Key Insights
The Biologics Contract Development and Manufacturing Organization (CDMO) market is experiencing robust growth, projected to reach \$15.32 billion in 2025 and exhibiting a Compound Annual Growth Rate (CAGR) of 12.78% from 2025 to 2033. This expansion is fueled by several key drivers. The increasing demand for biologics, driven by the rising prevalence of chronic diseases and the development of novel therapeutic modalities such as cell and gene therapies, is a primary factor. Furthermore, the growing outsourcing trend among pharmaceutical and biotechnology companies, seeking to reduce capital expenditure and leverage the expertise of specialized CDMOs, significantly contributes to market growth. Technological advancements in bioprocessing, leading to increased efficiency and reduced manufacturing costs, also play a crucial role. The market is segmented by product type (e.g., monoclonal antibodies, recombinant proteins, vaccines), by type (mammalian and microbial cell lines), and by other biologics including biosimilars. Competition is intense, with major players such as Samsung Biologics, Lonza, and Wuxi Biologics vying for market share, along with established pharmaceutical companies offering contract manufacturing services. Geographic expansion is also a significant trend, with regions like Asia Pacific witnessing particularly rapid growth due to increasing investments in biopharmaceutical infrastructure and a growing domestic market.
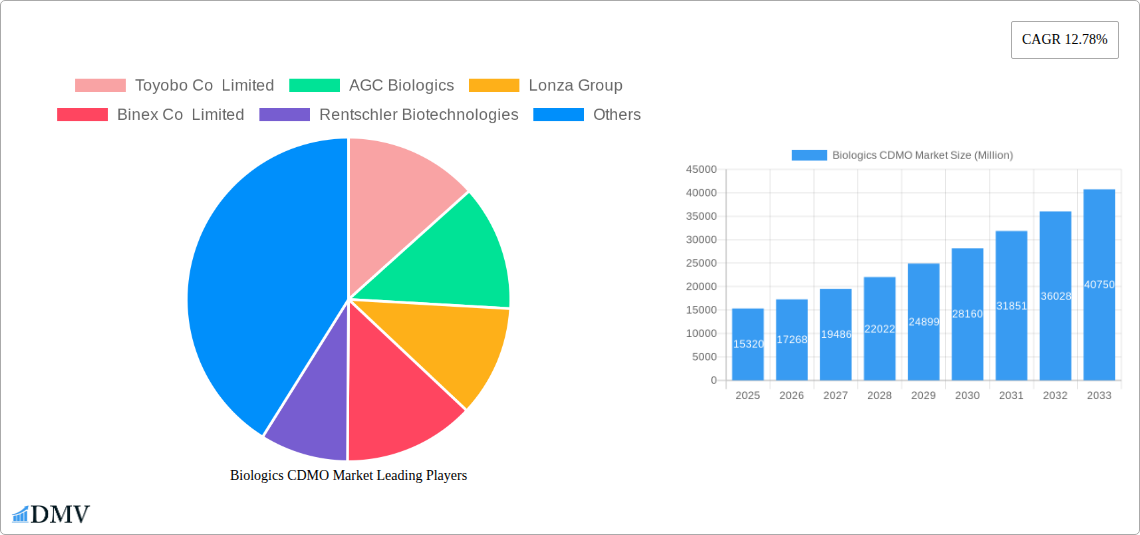
Biologics CDMO Market Market Size (In Billion)

While the market enjoys significant tailwinds, challenges remain. Regulatory hurdles and stringent quality control requirements can pose obstacles for smaller CDMOs. Furthermore, the high cost of biologics manufacturing and the need for specialized expertise can limit market penetration in certain regions. Despite these constraints, the long-term outlook for the Biologics CDMO market remains highly positive, driven by the continuous innovation in biologics therapeutics and the increasing reliance on outsourcing by pharmaceutical and biotechnology companies globally. The industry's ongoing investments in advanced technologies, coupled with strategic partnerships and acquisitions, will further shape the competitive landscape and fuel future growth.
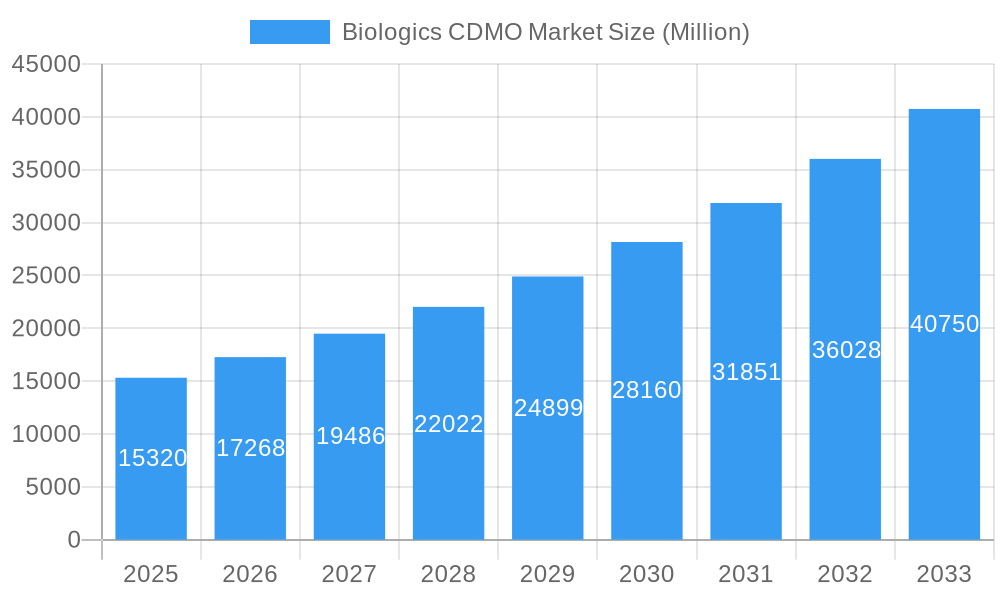
Biologics CDMO Market Company Market Share

This comprehensive report delivers an in-depth analysis of the Biologics CDMO Market, projecting a robust growth trajectory from 2025 to 2033. With a meticulous examination of the historical period (2019-2024), base year (2025), and estimated year (2025), this report offers crucial insights for stakeholders seeking to navigate this dynamic landscape. The study period spans 2019-2033, providing a long-term perspective on market trends and opportunities. The report's findings are invaluable for informed decision-making within the biologics contract development and manufacturing organization (CDMO) sector.
Biologics CDMO Market Market Composition & Trends
The Biologics CDMO market is a dynamic and evolving sector characterized by a moderately concentrated landscape. Leading players such as Wuxi Biologics, Samsung Biologics, Lonza Group, and Catalent Inc. command significant market share, yet the ecosystem is vibrant with numerous specialized CDMOs fostering a competitive environment. Innovation is a key differentiator, driven by rapid advancements in critical areas including sophisticated cell line engineering for enhanced productivity, process intensification techniques for optimized yields and efficiency, and cutting-edge analytical technologies essential for the production of increasingly complex biologics. The market operates within stringent regulatory frameworks, particularly in key regions like North America and Europe, which significantly influence manufacturing standards, quality control, and compliance protocols.
While chemically synthesized drugs represent a substitute product category, the inherent advantages and growing complexity of therapeutic molecules continue to drive robust demand for biologics. The primary end-users of Biologics CDMO services are pharmaceutical and biotechnology companies engaged in research and development across a wide spectrum of therapeutic areas. The industry is also marked by substantial Mergers and Acquisitions (M&A) activities. In recent years, deal values have exceeded significant figures, underscoring a trend towards industry consolidation and strategic expansion. Notable examples include FUJIFILM Corporation's acquisition of Atara Biotherapeutics Inc.'s cell therapy facility, which bolsters its manufacturing capabilities, and the strategic partnership established between AstraZeneca and Samsung Biologics to enhance biopharmaceutical production.
- Market Concentration: The market is moderately concentrated, with a few major players holding substantial market influence.
- Innovation Catalysts: Key drivers of innovation include advancements in cell line engineering, process intensification methodologies, and sophisticated analytical technologies.
- Regulatory Landscape: Stringent regulatory requirements heavily impact manufacturing practices and compliance, with North America and Europe being particularly influential regions.
- Substitute Products: Chemically synthesized drugs present a competitive alternative, though the unique benefits of biologics sustain their market growth.
- End-User Profile: The primary consumers of CDMO services are pharmaceutical and biotechnology firms across diverse therapeutic domains.
- M&A Activities: Significant M&A activity, with substantial annual deal values, highlights ongoing industry consolidation and strategic growth initiatives.
Biologics CDMO Market Industry Evolution
The Biologics CDMO market has undergone a transformative evolution, significantly shaped by technological breakthroughs and evolving market demands. Historically, from 2019 to 2024, the market demonstrated a robust Compound Annual Growth Rate (CAGR) of approximately xx%, primarily fueled by the escalating demand for biologics, particularly for treatments in oncology, immunology, and infectious diseases. Key technological advancements, such as the widespread adoption of single-use technologies for flexibility and reduced contamination risk, the implementation of continuous manufacturing for enhanced efficiency, and the integration of Process Analytical Technology (PAT) for real-time quality monitoring, have collectively contributed to improved operational efficiency, cost reductions, and superior product quality.
The anticipated trajectory for the forecast period (2025-2033) suggests a sustained CAGR of xx%, driven by the ongoing integration of these advanced manufacturing technologies. Consumer demand is increasingly leaning towards highly personalized and targeted therapeutic approaches, which in turn necessitates specialized CDMOs possessing deep expertise in manufacturing complex biologics. The burgeoning market for biosimilars is also a significant contributor to overall market expansion, with CDMOs playing an indispensable role in their development and large-scale production. Projections indicate that the Biologics CDMO market is set to reach a substantial valuation of xx Million by 2033, propelled by the enduring growth of biologic therapies and the continuous adoption of innovative manufacturing solutions.
Leading Regions, Countries, or Segments in Biologics CDMO Market
Currently, North America stands as the preeminent region in the Biologics CDMO market. This dominance is underpinned by its exceptionally strong pharmaceutical and biotechnology sectors, substantial investments in research and development (R&D), and a mature and well-established regulatory framework that supports innovation and compliance. Simultaneously, the Asia Pacific region is exhibiting remarkable growth. This rapid expansion is fueled by increasing healthcare expenditures, the burgeoning biopharmaceutical industries, and a growing patient population seeking advanced medical treatments. Government initiatives and a focus on expanding manufacturing capacities are also significant contributors to this regional surge.
Segment Analysis:
- By Type: Mammalian cell-derived biologics currently dominate the market due to their proven capability in producing complex and highly effective protein-based therapeutics. However, there is a notable increase in the utilization of non-mammalian (microbial) systems, which offer cost-effectiveness and scalability for specific product types.
- By Product Type: Monoclonal antibodies (mAbs) continue to hold the largest market share, reflecting their widespread therapeutic applications. Nevertheless, the demand for a broader range of biologics, including recombinant proteins, vaccines, and advanced cell and gene therapies, is on a significant upward trend.
- Other Biologics: Biosimilars: This segment is experiencing accelerated growth, driven by the expiry of patents for originator biologic drugs and the increasing availability and affordability of biosimilar alternatives, making advanced therapies more accessible to a wider patient population.
Biologics CDMO Market Product Innovations
Recent product innovations focus on enhancing the efficiency and scalability of biologics manufacturing. This includes the development of advanced cell lines with higher productivity, the implementation of continuous manufacturing processes, and the use of innovative analytical tools for real-time process monitoring. These innovations translate into reduced production costs, improved product quality, and faster time-to-market for new biologics. Unique selling propositions center on customized solutions, advanced technologies, and stringent quality standards.
Propelling Factors for Biologics CDMO Market Growth
Several key factors are driving the growth of the Biologics CDMO market. Technological advancements in areas like cell line engineering and process intensification are significantly improving efficiency and reducing production costs. Favorable regulatory environments, particularly in major markets such as North America and Europe, encourage innovation and investment. The increased demand for biologics, spurred by the rising prevalence of chronic diseases and the development of innovative therapies, fuels market expansion. Finally, strategic partnerships and mergers and acquisitions (M&A) activities consolidate market share and drive further growth.
Obstacles in the Biologics CDMO Market Market
The Biologics CDMO market faces several challenges. Stringent regulatory requirements can increase production costs and lead to delays in product launches. Supply chain disruptions, particularly for raw materials and specialized equipment, can impact manufacturing timelines and output. Intense competition among established and emerging players creates pricing pressure. The rising cost of development and manufacturing adds to the complexities and increases risk.
Future Opportunities in Biologics CDMO Market
Future opportunities lie in the expansion into emerging markets, focusing on personalized medicine, and the development of advanced therapies like cell and gene therapies. Innovations in process development and manufacturing technologies, particularly continuous manufacturing and automation, will enhance efficiency and reduce costs. The increasing demand for biosimilars presents substantial growth potential for CDMOs specializing in biosimilar manufacturing.
Major Players in the Biologics CDMO Market Ecosystem
- Toyobo Co Limited
- AGC Biologics
- Lonza Group
- Binex Co Limited
- Rentschler Biotechnologies
- Wuxi Biologics
- AbbVie Contract Manufacturing
- Parexel International Corporation
- Sandoz Biopharmaceuticals (Novartis AG)
- Catalent Inc
- JRS Pharma
- Fujifilm Diosynth Biotechnologies USA Inc
- Samsung Biologics
- Boehringer Ingelheim Group
- Icon PLC
Key Developments in Biologics CDMO Market Industry
- December 2021: AstraZeneca and Samsung Biologics strengthened their alliance by entering into a significant strategic biopharmaceutical manufacturing partnership. This expansion included the production of a cancer immunotherapy product, valued at approximately USD 380 Million, highlighting the growing collaboration in advanced therapeutic manufacturing.
- March 2022: Oasmia Pharmaceutical AB and Lonza finalized a substantial large-scale manufacturing agreement. This collaboration focuses on the production of a drug intermediate, ensuring the supply of critical clinical materials for Cantrixil, thereby supporting its development pathway.
- April 2022: FUJIFILM Corporation enhanced its global biopharmaceutical manufacturing footprint through the acquisition of a dedicated cell therapy manufacturing facility from Atara Biotherapeutics Inc. This strategic move significantly expands the capabilities of FUJIFILM Diosynth Biotechnologies in the rapidly growing cell therapy sector.
Strategic Biologics CDMO Market Market Forecast
The Biologics CDMO market is poised for sustained growth, driven by technological innovation, increasing demand for biologics, and expansion into emerging markets. The forecast period (2025-2033) anticipates significant market expansion, exceeding xx Million, fueled by the rising adoption of advanced therapies and the ongoing consolidation of the industry. The potential for increased efficiency, reduced production costs, and improved product quality offers significant growth opportunities for CDMOs that embrace technological advancements and strategic partnerships.
Biologics CDMO Market Segmentation
-
1. Type
- 1.1. Mammalian
- 1.2. Non-mammalian (Microbial)
-
2. Product Type
-
2.1. Biologics
- 2.1.1. Monoclon
- 2.1.2. Recombinant Proteins
- 2.1.3. Antisense and Molecular Therapy
- 2.1.4. Vaccines
- 2.1.5. Other Biologics
- 2.2. Biosimilars
-
2.1. Biologics
Biologics CDMO Market Segmentation By Geography
- 1. North America
- 2. Europe
- 3. Asia
- 4. Australia and New Zealand
- 5. Latin America
- 6. Middle East and Africa
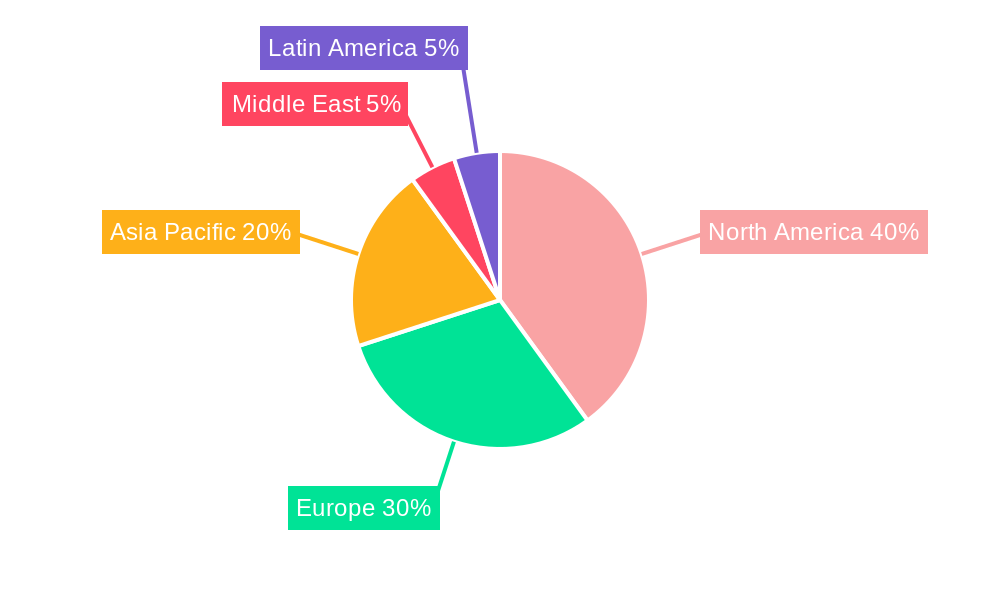
Biologics CDMO Market Regional Market Share

Geographic Coverage of Biologics CDMO Market
Biologics CDMO Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 12.78% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Access to New Technologies and Higher Speed of Execution Realized by CDMOs; Need for High Capital Investments to Develop Capabilities Has Led to Firms Choosing the Outsourcing Model; Lack of In-house Capacity among Emerging Drug Development Companies
- 3.3. Market Restrains
- 3.3.1. Presence of Alternative Printing Technology
- 3.4. Market Trends
- 3.4.1. CDMOs’ Access to New Technologies and Higher Speed of Execution Driving Market Growth
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Biologics CDMO Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Type
- 5.1.1. Mammalian
- 5.1.2. Non-mammalian (Microbial)
- 5.2. Market Analysis, Insights and Forecast - by Product Type
- 5.2.1. Biologics
- 5.2.1.1. Monoclon
- 5.2.1.2. Recombinant Proteins
- 5.2.1.3. Antisense and Molecular Therapy
- 5.2.1.4. Vaccines
- 5.2.1.5. Other Biologics
- 5.2.2. Biosimilars
- 5.2.1. Biologics
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. Europe
- 5.3.3. Asia
- 5.3.4. Australia and New Zealand
- 5.3.5. Latin America
- 5.3.6. Middle East and Africa
- 5.1. Market Analysis, Insights and Forecast - by Type
- 6. North America Biologics CDMO Market Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Type
- 6.1.1. Mammalian
- 6.1.2. Non-mammalian (Microbial)
- 6.2. Market Analysis, Insights and Forecast - by Product Type
- 6.2.1. Biologics
- 6.2.1.1. Monoclon
- 6.2.1.2. Recombinant Proteins
- 6.2.1.3. Antisense and Molecular Therapy
- 6.2.1.4. Vaccines
- 6.2.1.5. Other Biologics
- 6.2.2. Biosimilars
- 6.2.1. Biologics
- 6.1. Market Analysis, Insights and Forecast - by Type
- 7. Europe Biologics CDMO Market Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Type
- 7.1.1. Mammalian
- 7.1.2. Non-mammalian (Microbial)
- 7.2. Market Analysis, Insights and Forecast - by Product Type
- 7.2.1. Biologics
- 7.2.1.1. Monoclon
- 7.2.1.2. Recombinant Proteins
- 7.2.1.3. Antisense and Molecular Therapy
- 7.2.1.4. Vaccines
- 7.2.1.5. Other Biologics
- 7.2.2. Biosimilars
- 7.2.1. Biologics
- 7.1. Market Analysis, Insights and Forecast - by Type
- 8. Asia Biologics CDMO Market Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Type
- 8.1.1. Mammalian
- 8.1.2. Non-mammalian (Microbial)
- 8.2. Market Analysis, Insights and Forecast - by Product Type
- 8.2.1. Biologics
- 8.2.1.1. Monoclon
- 8.2.1.2. Recombinant Proteins
- 8.2.1.3. Antisense and Molecular Therapy
- 8.2.1.4. Vaccines
- 8.2.1.5. Other Biologics
- 8.2.2. Biosimilars
- 8.2.1. Biologics
- 8.1. Market Analysis, Insights and Forecast - by Type
- 9. Australia and New Zealand Biologics CDMO Market Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Type
- 9.1.1. Mammalian
- 9.1.2. Non-mammalian (Microbial)
- 9.2. Market Analysis, Insights and Forecast - by Product Type
- 9.2.1. Biologics
- 9.2.1.1. Monoclon
- 9.2.1.2. Recombinant Proteins
- 9.2.1.3. Antisense and Molecular Therapy
- 9.2.1.4. Vaccines
- 9.2.1.5. Other Biologics
- 9.2.2. Biosimilars
- 9.2.1. Biologics
- 9.1. Market Analysis, Insights and Forecast - by Type
- 10. Latin America Biologics CDMO Market Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Type
- 10.1.1. Mammalian
- 10.1.2. Non-mammalian (Microbial)
- 10.2. Market Analysis, Insights and Forecast - by Product Type
- 10.2.1. Biologics
- 10.2.1.1. Monoclon
- 10.2.1.2. Recombinant Proteins
- 10.2.1.3. Antisense and Molecular Therapy
- 10.2.1.4. Vaccines
- 10.2.1.5. Other Biologics
- 10.2.2. Biosimilars
- 10.2.1. Biologics
- 10.1. Market Analysis, Insights and Forecast - by Type
- 11. Middle East and Africa Biologics CDMO Market Analysis, Insights and Forecast, 2020-2032
- 11.1. Market Analysis, Insights and Forecast - by Type
- 11.1.1. Mammalian
- 11.1.2. Non-mammalian (Microbial)
- 11.2. Market Analysis, Insights and Forecast - by Product Type
- 11.2.1. Biologics
- 11.2.1.1. Monoclon
- 11.2.1.2. Recombinant Proteins
- 11.2.1.3. Antisense and Molecular Therapy
- 11.2.1.4. Vaccines
- 11.2.1.5. Other Biologics
- 11.2.2. Biosimilars
- 11.2.1. Biologics
- 11.1. Market Analysis, Insights and Forecast - by Type
- 12. Competitive Analysis
- 12.1. Global Market Share Analysis 2025
- 12.2. Company Profiles
- 12.2.1 Toyobo Co Limited
- 12.2.1.1. Overview
- 12.2.1.2. Products
- 12.2.1.3. SWOT Analysis
- 12.2.1.4. Recent Developments
- 12.2.1.5. Financials (Based on Availability)
- 12.2.2 AGC Biologics
- 12.2.2.1. Overview
- 12.2.2.2. Products
- 12.2.2.3. SWOT Analysis
- 12.2.2.4. Recent Developments
- 12.2.2.5. Financials (Based on Availability)
- 12.2.3 Lonza Group
- 12.2.3.1. Overview
- 12.2.3.2. Products
- 12.2.3.3. SWOT Analysis
- 12.2.3.4. Recent Developments
- 12.2.3.5. Financials (Based on Availability)
- 12.2.4 Binex Co Limited
- 12.2.4.1. Overview
- 12.2.4.2. Products
- 12.2.4.3. SWOT Analysis
- 12.2.4.4. Recent Developments
- 12.2.4.5. Financials (Based on Availability)
- 12.2.5 Rentschler Biotechnologies
- 12.2.5.1. Overview
- 12.2.5.2. Products
- 12.2.5.3. SWOT Analysis
- 12.2.5.4. Recent Developments
- 12.2.5.5. Financials (Based on Availability)
- 12.2.6 Wuxi Biologics
- 12.2.6.1. Overview
- 12.2.6.2. Products
- 12.2.6.3. SWOT Analysis
- 12.2.6.4. Recent Developments
- 12.2.6.5. Financials (Based on Availability)
- 12.2.7 AbbVie Contract Manufacturing*List Not Exhaustive
- 12.2.7.1. Overview
- 12.2.7.2. Products
- 12.2.7.3. SWOT Analysis
- 12.2.7.4. Recent Developments
- 12.2.7.5. Financials (Based on Availability)
- 12.2.8 Parexel International Corporation
- 12.2.8.1. Overview
- 12.2.8.2. Products
- 12.2.8.3. SWOT Analysis
- 12.2.8.4. Recent Developments
- 12.2.8.5. Financials (Based on Availability)
- 12.2.9 Sandoz Biopharmaceuticals (Novartis AG)
- 12.2.9.1. Overview
- 12.2.9.2. Products
- 12.2.9.3. SWOT Analysis
- 12.2.9.4. Recent Developments
- 12.2.9.5. Financials (Based on Availability)
- 12.2.10 Catalent Inc
- 12.2.10.1. Overview
- 12.2.10.2. Products
- 12.2.10.3. SWOT Analysis
- 12.2.10.4. Recent Developments
- 12.2.10.5. Financials (Based on Availability)
- 12.2.11 JRS Pharma
- 12.2.11.1. Overview
- 12.2.11.2. Products
- 12.2.11.3. SWOT Analysis
- 12.2.11.4. Recent Developments
- 12.2.11.5. Financials (Based on Availability)
- 12.2.12 Fujifilm Diosynth Biotechnologies USA Inc
- 12.2.12.1. Overview
- 12.2.12.2. Products
- 12.2.12.3. SWOT Analysis
- 12.2.12.4. Recent Developments
- 12.2.12.5. Financials (Based on Availability)
- 12.2.13 Samsung Biologics
- 12.2.13.1. Overview
- 12.2.13.2. Products
- 12.2.13.3. SWOT Analysis
- 12.2.13.4. Recent Developments
- 12.2.13.5. Financials (Based on Availability)
- 12.2.14 Boehringer Ingelheim Group
- 12.2.14.1. Overview
- 12.2.14.2. Products
- 12.2.14.3. SWOT Analysis
- 12.2.14.4. Recent Developments
- 12.2.14.5. Financials (Based on Availability)
- 12.2.15 Icon PLC
- 12.2.15.1. Overview
- 12.2.15.2. Products
- 12.2.15.3. SWOT Analysis
- 12.2.15.4. Recent Developments
- 12.2.15.5. Financials (Based on Availability)
- 12.2.1 Toyobo Co Limited
List of Figures
- Figure 1: Global Biologics CDMO Market Revenue Breakdown (Million, %) by Region 2025 & 2033
- Figure 2: North America Biologics CDMO Market Revenue (Million), by Type 2025 & 2033
- Figure 3: North America Biologics CDMO Market Revenue Share (%), by Type 2025 & 2033
- Figure 4: North America Biologics CDMO Market Revenue (Million), by Product Type 2025 & 2033
- Figure 5: North America Biologics CDMO Market Revenue Share (%), by Product Type 2025 & 2033
- Figure 6: North America Biologics CDMO Market Revenue (Million), by Country 2025 & 2033
- Figure 7: North America Biologics CDMO Market Revenue Share (%), by Country 2025 & 2033
- Figure 8: Europe Biologics CDMO Market Revenue (Million), by Type 2025 & 2033
- Figure 9: Europe Biologics CDMO Market Revenue Share (%), by Type 2025 & 2033
- Figure 10: Europe Biologics CDMO Market Revenue (Million), by Product Type 2025 & 2033
- Figure 11: Europe Biologics CDMO Market Revenue Share (%), by Product Type 2025 & 2033
- Figure 12: Europe Biologics CDMO Market Revenue (Million), by Country 2025 & 2033
- Figure 13: Europe Biologics CDMO Market Revenue Share (%), by Country 2025 & 2033
- Figure 14: Asia Biologics CDMO Market Revenue (Million), by Type 2025 & 2033
- Figure 15: Asia Biologics CDMO Market Revenue Share (%), by Type 2025 & 2033
- Figure 16: Asia Biologics CDMO Market Revenue (Million), by Product Type 2025 & 2033
- Figure 17: Asia Biologics CDMO Market Revenue Share (%), by Product Type 2025 & 2033
- Figure 18: Asia Biologics CDMO Market Revenue (Million), by Country 2025 & 2033
- Figure 19: Asia Biologics CDMO Market Revenue Share (%), by Country 2025 & 2033
- Figure 20: Australia and New Zealand Biologics CDMO Market Revenue (Million), by Type 2025 & 2033
- Figure 21: Australia and New Zealand Biologics CDMO Market Revenue Share (%), by Type 2025 & 2033
- Figure 22: Australia and New Zealand Biologics CDMO Market Revenue (Million), by Product Type 2025 & 2033
- Figure 23: Australia and New Zealand Biologics CDMO Market Revenue Share (%), by Product Type 2025 & 2033
- Figure 24: Australia and New Zealand Biologics CDMO Market Revenue (Million), by Country 2025 & 2033
- Figure 25: Australia and New Zealand Biologics CDMO Market Revenue Share (%), by Country 2025 & 2033
- Figure 26: Latin America Biologics CDMO Market Revenue (Million), by Type 2025 & 2033
- Figure 27: Latin America Biologics CDMO Market Revenue Share (%), by Type 2025 & 2033
- Figure 28: Latin America Biologics CDMO Market Revenue (Million), by Product Type 2025 & 2033
- Figure 29: Latin America Biologics CDMO Market Revenue Share (%), by Product Type 2025 & 2033
- Figure 30: Latin America Biologics CDMO Market Revenue (Million), by Country 2025 & 2033
- Figure 31: Latin America Biologics CDMO Market Revenue Share (%), by Country 2025 & 2033
- Figure 32: Middle East and Africa Biologics CDMO Market Revenue (Million), by Type 2025 & 2033
- Figure 33: Middle East and Africa Biologics CDMO Market Revenue Share (%), by Type 2025 & 2033
- Figure 34: Middle East and Africa Biologics CDMO Market Revenue (Million), by Product Type 2025 & 2033
- Figure 35: Middle East and Africa Biologics CDMO Market Revenue Share (%), by Product Type 2025 & 2033
- Figure 36: Middle East and Africa Biologics CDMO Market Revenue (Million), by Country 2025 & 2033
- Figure 37: Middle East and Africa Biologics CDMO Market Revenue Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Biologics CDMO Market Revenue Million Forecast, by Type 2020 & 2033
- Table 2: Global Biologics CDMO Market Revenue Million Forecast, by Product Type 2020 & 2033
- Table 3: Global Biologics CDMO Market Revenue Million Forecast, by Region 2020 & 2033
- Table 4: Global Biologics CDMO Market Revenue Million Forecast, by Type 2020 & 2033
- Table 5: Global Biologics CDMO Market Revenue Million Forecast, by Product Type 2020 & 2033
- Table 6: Global Biologics CDMO Market Revenue Million Forecast, by Country 2020 & 2033
- Table 7: Global Biologics CDMO Market Revenue Million Forecast, by Type 2020 & 2033
- Table 8: Global Biologics CDMO Market Revenue Million Forecast, by Product Type 2020 & 2033
- Table 9: Global Biologics CDMO Market Revenue Million Forecast, by Country 2020 & 2033
- Table 10: Global Biologics CDMO Market Revenue Million Forecast, by Type 2020 & 2033
- Table 11: Global Biologics CDMO Market Revenue Million Forecast, by Product Type 2020 & 2033
- Table 12: Global Biologics CDMO Market Revenue Million Forecast, by Country 2020 & 2033
- Table 13: Global Biologics CDMO Market Revenue Million Forecast, by Type 2020 & 2033
- Table 14: Global Biologics CDMO Market Revenue Million Forecast, by Product Type 2020 & 2033
- Table 15: Global Biologics CDMO Market Revenue Million Forecast, by Country 2020 & 2033
- Table 16: Global Biologics CDMO Market Revenue Million Forecast, by Type 2020 & 2033
- Table 17: Global Biologics CDMO Market Revenue Million Forecast, by Product Type 2020 & 2033
- Table 18: Global Biologics CDMO Market Revenue Million Forecast, by Country 2020 & 2033
- Table 19: Global Biologics CDMO Market Revenue Million Forecast, by Type 2020 & 2033
- Table 20: Global Biologics CDMO Market Revenue Million Forecast, by Product Type 2020 & 2033
- Table 21: Global Biologics CDMO Market Revenue Million Forecast, by Country 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Biologics CDMO Market?
The projected CAGR is approximately 12.78%.
2. Which companies are prominent players in the Biologics CDMO Market?
Key companies in the market include Toyobo Co Limited, AGC Biologics, Lonza Group, Binex Co Limited, Rentschler Biotechnologies, Wuxi Biologics, AbbVie Contract Manufacturing*List Not Exhaustive, Parexel International Corporation, Sandoz Biopharmaceuticals (Novartis AG), Catalent Inc, JRS Pharma, Fujifilm Diosynth Biotechnologies USA Inc, Samsung Biologics, Boehringer Ingelheim Group, Icon PLC.
3. What are the main segments of the Biologics CDMO Market?
The market segments include Type, Product Type.
4. Can you provide details about the market size?
The market size is estimated to be USD 15.32 Million as of 2022.
5. What are some drivers contributing to market growth?
Access to New Technologies and Higher Speed of Execution Realized by CDMOs; Need for High Capital Investments to Develop Capabilities Has Led to Firms Choosing the Outsourcing Model; Lack of In-house Capacity among Emerging Drug Development Companies.
6. What are the notable trends driving market growth?
CDMOs’ Access to New Technologies and Higher Speed of Execution Driving Market Growth.
7. Are there any restraints impacting market growth?
Presence of Alternative Printing Technology.
8. Can you provide examples of recent developments in the market?
April 2022 - FUJIFILM Corporation announced that it had completed the acquisition of a dedicated cell therapy manufacturing facility from Atara Biotherapeutics Inc. The facility, located in Thousand Oaks, California, will be operated as part of FUJIFILM DiosynthBiotechnologies' global network, a subsidiary of FUJIFILM Corporation and a world-leading contract development and manufacturing organization (CDMO).
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Biologics CDMO Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Biologics CDMO Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Biologics CDMO Market?
To stay informed about further developments, trends, and reports in the Biologics CDMO Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database
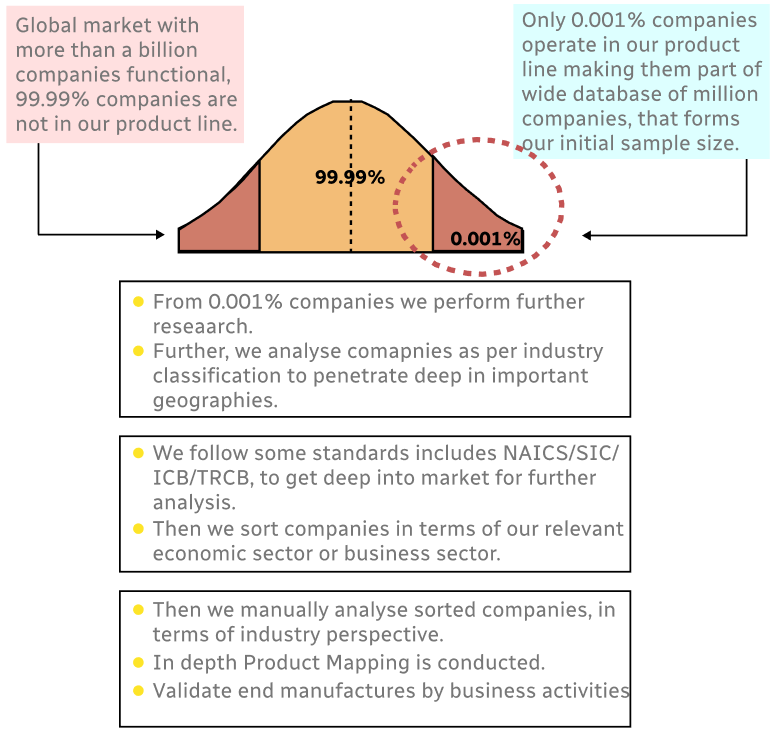
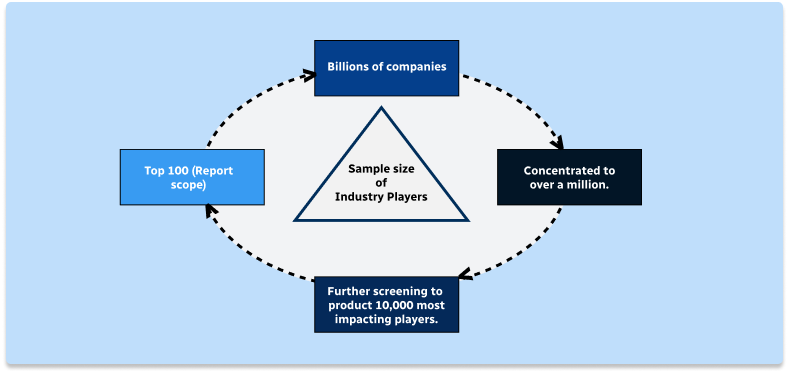
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
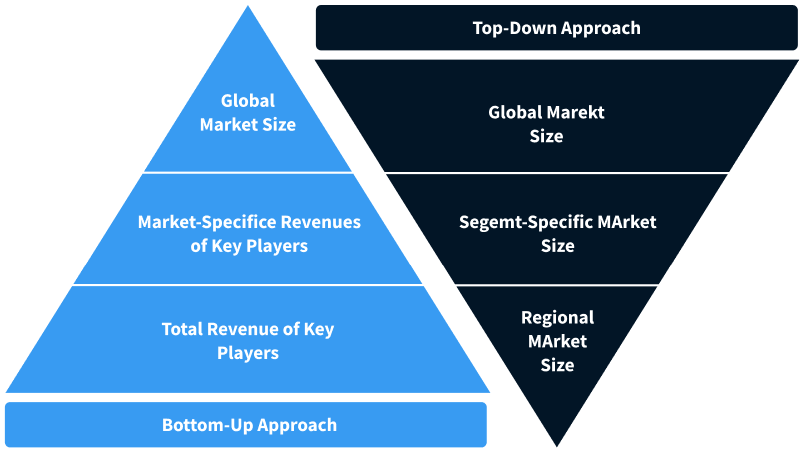
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
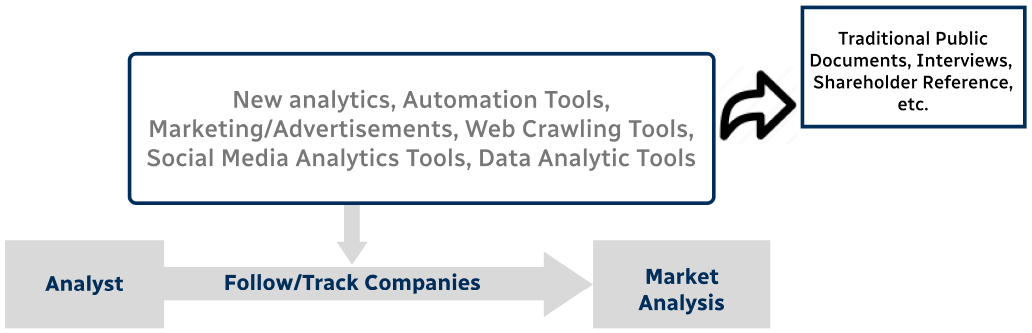
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


