Key Insights
The United States Neonatal and Prenatal Devices Market is poised for significant expansion, projected to reach $5.4 billion in 2020 with a robust Compound Annual Growth Rate (CAGR) of 6.1% over the forecast period. This growth trajectory is primarily fueled by an increasing awareness of fetal and neonatal health, coupled with advancements in medical technology that enable earlier and more accurate diagnostics and interventions. The rising incidence of premature births and complex pregnancies, alongside supportive government initiatives and increased healthcare spending, are key drivers propelling market demand. Furthermore, a growing emphasis on personalized medicine and the development of innovative, user-friendly devices are contributing to the market's upward trend. The market is segmented into Prenatal and Fetal Equipment, encompassing a wide range of diagnostic and monitoring tools like ultrasound, fetal Doppler, and MRI, and Neonatal Equipment, which includes vital devices such as incubators, monitoring systems, and respiratory support machines.
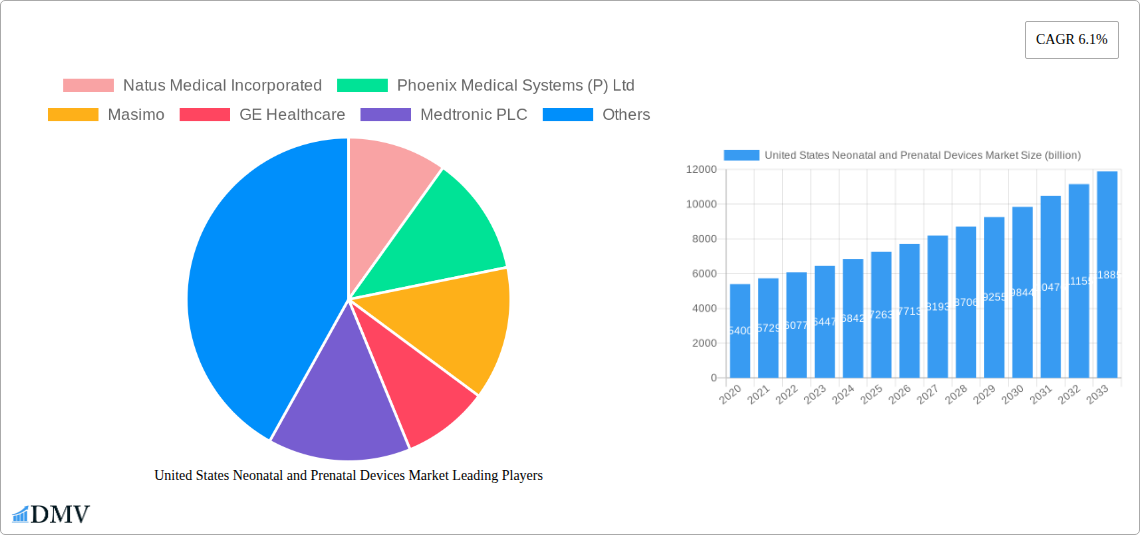
United States Neonatal and Prenatal Devices Market Market Size (In Billion)

Navigating the projected growth, the market encounters certain restraints that warrant strategic consideration. The high cost of advanced neonatal and prenatal devices, coupled with the need for specialized training for healthcare professionals to operate them effectively, can pose significant barriers to widespread adoption, particularly in smaller healthcare facilities. Additionally, stringent regulatory approvals for medical devices add to the time and cost associated with bringing new technologies to market. However, the unwavering commitment to improving infant and maternal outcomes, coupled with ongoing research and development by leading companies like GE Healthcare, Philips, and Medtronic, is expected to overcome these challenges. Emerging trends such as the integration of artificial intelligence for enhanced diagnostic capabilities and the development of portable, connected devices for remote monitoring are set to redefine the market landscape, offering new avenues for growth and innovation in the United States.
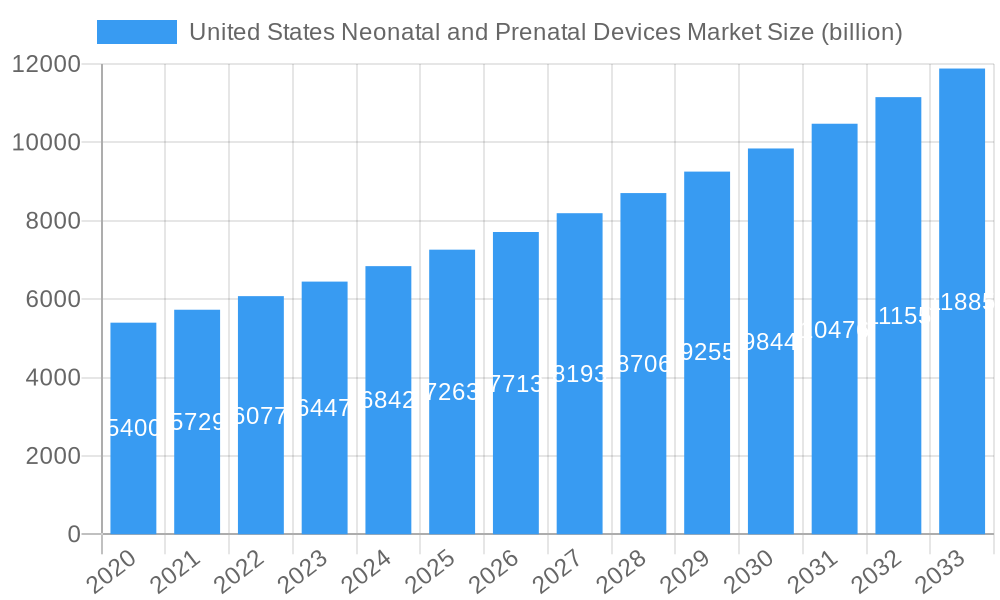
United States Neonatal and Prenatal Devices Market Company Market Share

Gain unparalleled insights into the dynamic United States neonatal and prenatal devices market, a sector critical for ensuring optimal maternal and infant health outcomes. This in-depth report analyzes market composition, industry evolution, regional dominance, product innovations, growth drivers, challenges, and future opportunities, providing a strategic roadmap for stakeholders. With a comprehensive study period spanning 2019–2033, a base and estimated year of 2025, and a forecast period from 2025–2033, this report offers data-driven projections and actionable intelligence. The estimated market size for the United States Neonatal and Prenatal Devices Market in 2025 is projected to reach $XX billion, with a projected Compound Annual Growth Rate (CAGR) of XX% during the forecast period, reaching an impressive $XX billion by 2033.
United States Neonatal and Prenatal Devices Market Market Composition & Trends
The United States neonatal and prenatal devices market is characterized by a moderate to high degree of market concentration, with key players like GE Healthcare, Medtronic PLC, and Koninklijke Philips NV holding significant market shares. Innovation is a primary catalyst for growth, driven by advancements in artificial intelligence, miniaturization, and non-invasive monitoring technologies, aiming to improve diagnostic accuracy and patient comfort for both fetuses and neonates. The regulatory landscape, governed by the FDA, plays a crucial role, with stringent approval processes shaping product development and market entry. Substitute products, while present in some niche areas, generally do not offer the comprehensive diagnostic and therapeutic capabilities of specialized neonatal and prenatal devices. End-user profiles are diverse, encompassing hospitals, specialized clinics, birthing centers, and research institutions, each with distinct purchasing drivers. Mergers and acquisitions (M&A) activity has been strategic, with deal values in the hundreds of millions of dollars, aimed at expanding product portfolios and market reach. Key M&A activities have focused on acquiring innovative technologies and enhancing distribution networks. The market share distribution reveals a competitive landscape where established players leverage their brand recognition and existing infrastructure, while newer entrants focus on disruptive technologies.
United States Neonatal and Prenatal Devices Market Industry Evolution
The United States neonatal and prenatal devices market has undergone a significant evolution, driven by a confluence of technological advancements, increasing awareness of fetal and neonatal health, and a growing demand for sophisticated diagnostic and therapeutic solutions. Over the historical period of 2019–2024, the market witnessed steady growth, fueled by rising birth rates in specific demographics and a heightened focus on reducing infant mortality and morbidity. The base year of 2025 represents a pivotal point, with the market projected to accelerate its growth trajectory. Technological innovations have been at the forefront of this evolution. The development of advanced ultrasound and ultrasonography devices has led to higher resolution imaging, enabling earlier and more accurate detection of fetal anomalies. Fetal heart monitors have become more sophisticated, offering continuous, real-time data and improved portability for outpatient monitoring. In the neonatal segment, incubators have evolved from basic environmental control to integrated monitoring and therapeutic systems, providing a more nurturing and responsive environment for premature and ill infants. Neonatal monitoring devices, including pulse oximeters and vital sign monitors, have become more accurate and less invasive, minimizing discomfort for vulnerable newborns. Respiratory assistance and monitoring devices, such as ventilators and CPAP systems, have seen significant advancements, offering more personalized and effective respiratory support. The forecast period of 2025–2033 is expected to witness an even more rapid expansion, driven by the increasing adoption of connected devices, AI-powered diagnostics, and remote patient monitoring solutions. Shifting consumer demands, particularly from expectant parents seeking comprehensive prenatal care and reassurance, also play a crucial role. This has led to a greater demand for user-friendly, portable, and accurate home-use prenatal devices. Furthermore, the growing prevalence of high-risk pregnancies and the increasing survival rates of extremely premature infants necessitate continuous innovation and the availability of advanced neonatal care equipment. The market’s growth trajectory is directly linked to the increasing investment in healthcare infrastructure and research and development by leading manufacturers.
Leading Regions, Countries, or Segments in United States Neonatal and Prenatal Devices Market
Within the United States neonatal and prenatal devices market, the Prenatal and Fetal Equipment segment demonstrably leads, driven by a powerful synergy of technological innovation, increasing demand for early diagnostics, and a strong emphasis on proactive maternal and fetal health management. This dominance is further amplified by the sub-segment of Ultrasound and Ultrasonography Devices, which forms the bedrock of prenatal screening and diagnosis. The widespread adoption of these devices across virtually all healthcare settings, from large hospital networks to independent imaging centers, underscores their critical role. Investment trends in this sub-segment are consistently high, with manufacturers pouring resources into developing higher resolution, more portable, and AI-enhanced ultrasound systems. Regulatory support, while stringent, prioritizes devices that improve diagnostic accuracy and patient safety, further bolstering the market for advanced ultrasound technology.
- Dominance of Prenatal and Fetal Equipment:
- Ultrasound and Ultrasonography Devices: This segment is the primary driver of market growth due to its indispensable role in routine prenatal care, anomaly detection, and guiding interventions. Increased demand for 3D and 4D imaging, coupled with advancements in Doppler technology for assessing blood flow, fuels continuous market expansion.
- Fetal Heart Monitors: Essential for monitoring fetal well-being during pregnancy and labor, these devices have seen significant technological upgrades, including wireless connectivity and remote monitoring capabilities, enhancing their utility and adoption rates.
- Fetal Doppler: While a more basic device, its accessibility and affordability make it a staple for routine check-ups and home monitoring, contributing to its steady market presence.
- Fetal Magnetic Resonance Imaging (MRI): Though a niche segment, fetal MRI is gaining traction for complex diagnostic challenges where ultrasound is insufficient, indicating a growing demand for advanced imaging solutions.
- Other Prenatal and Fetal Equipment: This includes a range of devices such as amniotic fluid monitoring systems and specialized imaging accessories that support the primary diagnostic tools.
In-depth analysis of this segment's dominance reveals that the proactive approach to pregnancy care in the United States, coupled with a robust healthcare reimbursement system, encourages early and frequent diagnostic testing. This creates a consistent demand for high-quality prenatal and fetal equipment. The increasing awareness among expectant parents about the benefits of early detection and monitoring also contributes significantly to market penetration. Furthermore, technological advancements that enhance portability and ease of use are expanding the reach of these devices beyond traditional hospital settings.
United States Neonatal and Prenatal Devices Market Product Innovations
Product innovation in the United States neonatal and prenatal devices market is rapidly advancing, focusing on enhancing diagnostic accuracy, improving patient comfort, and enabling seamless data integration. Manufacturers are developing more compact and user-friendly prenatal ultrasound systems, such as the Philips Ultrasound Compact system, which received FDA 510(k) clearance in September 2022 for its optimized portability and performance. In neonatal care, innovations include advanced incubators with integrated monitoring and therapeutic capabilities, and less invasive respiratory support devices. The development of AI-powered diagnostic tools that can analyze imaging data and patient vitals for early detection of critical conditions is a significant trend. Furthermore, the advent of devices like Maternova Inc.'s Preemie Test, which accurately assesses gestational age, offers a crucial advancement for neonatal survival by enabling timely and appropriate interventions. These innovations are characterized by improved performance metrics, reduced risk of complications, and enhanced patient outcomes, driving market adoption and expanding the application scope of these essential medical technologies.
Propelling Factors for United States Neonatal and Prenatal Devices Market Growth
Several key factors are propelling the growth of the United States neonatal and prenatal devices market. Technologically, continuous innovation in imaging, monitoring, and respiratory support systems is driving demand for advanced solutions. The increasing prevalence of high-risk pregnancies and premature births necessitates sophisticated equipment for optimal care. Economically, rising healthcare expenditure and favorable reimbursement policies for diagnostic and therapeutic procedures support market expansion. Regulatory bodies, such as the FDA, play a role by approving innovative devices that demonstrate improved safety and efficacy, further encouraging market adoption. The growing awareness among expectant parents regarding the importance of prenatal and neonatal care also contributes significantly to increased demand for these devices.
Obstacles in the United States Neonatal and Prenatal Devices Market Market
Despite robust growth, the United States neonatal and prenatal devices market faces certain obstacles. Stringent regulatory approval processes, while essential for patient safety, can lead to extended product launch timelines and increased development costs for manufacturers. The high cost of advanced medical devices can also pose a barrier to adoption, particularly for smaller healthcare facilities or in underserved regions. Supply chain disruptions, as evidenced by recent global events, can impact the availability and pricing of critical components. Furthermore, intense competition among established players and emerging market entrants can exert downward pressure on profit margins, requiring continuous innovation and cost-effective manufacturing strategies.
Future Opportunities in United States Neonatal and Prenatal Devices Market
The United States neonatal and prenatal devices market presents significant future opportunities. The growing adoption of telehealth and remote patient monitoring technologies opens avenues for innovative prenatal and neonatal monitoring solutions that can be used in home settings. Advancements in AI and machine learning are creating opportunities for predictive diagnostics and personalized treatment plans. The increasing demand for minimally invasive procedures in both prenatal diagnostics and neonatal care will drive the development of new tools and techniques. Furthermore, expanding into emerging markets with growing healthcare infrastructure and increasing disposable incomes offers substantial growth potential for manufacturers.
Major Players in the United States Neonatal and Prenatal Devices Market Ecosystem
- Natus Medical Incorporated
- Phoenix Medical Systems (P) Ltd
- Masimo
- GE Healthcare
- Medtronic PLC
- Vyaire Medical
- Getinge AB
- Koninklijke Philips NV
Key Developments in United States Neonatal and Prenatal Devices Market Industry
- September 2022: Philips received FDA 510(k) clearance for the Ultrasound Compact system to optimize portability and performance.
- July 2022: Maternova Inc. announced that it had signed an agreement with BirthTech Lda, Portugal, to distribute its Preemie Test in the United States, Europe, and Asia. The Preemie Test is the first medical device clinically proven to accurately assess the gestational age of a newborn, which is the major marker of neonatal survival.
Strategic United States Neonatal and Prenatal Devices Market Market Forecast
The United States neonatal and prenatal devices market is poised for substantial growth, driven by a strategic combination of technological advancements, increasing healthcare investments, and a growing emphasis on preventative maternal and infant care. The forecast period of 2025–2033 anticipates a significant expansion fueled by the continuous integration of artificial intelligence into diagnostic imaging and monitoring systems, enhancing predictive capabilities and accuracy. The increasing adoption of portable and connected devices will further broaden access to critical prenatal and neonatal healthcare, especially in remote and underserved areas. Future opportunities lie in developing more sophisticated, non-invasive monitoring technologies and personalized therapeutic solutions. The market’s trajectory is robust, promising continued innovation and improved health outcomes for mothers and newborns.
United States Neonatal and Prenatal Devices Market Segmentation
-
1. Product Type
-
1.1. Prenatal and Fetal Equipment
- 1.1.1. Ultrasound and Ultrasonography Devices
- 1.1.2. Fetal Doppler
- 1.1.3. Fetal Magnetic Resonance Imaging (MRI)
- 1.1.4. Fetal Heart Monitors
- 1.1.5. Fetal Pulse Oximeters
- 1.1.6. Other Prenatal and Fetal Equipment
-
1.2. Neonatal Equipment
- 1.2.1. Incubators
- 1.2.2. Neonatal Monitoring Devices
- 1.2.3. Phototherapy Equipment
- 1.2.4. Respiratory Assistance and Monitoring Devices
- 1.2.5. Other Neonatal Care Equipment
-
1.1. Prenatal and Fetal Equipment
United States Neonatal and Prenatal Devices Market Segmentation By Geography
- 1. United States
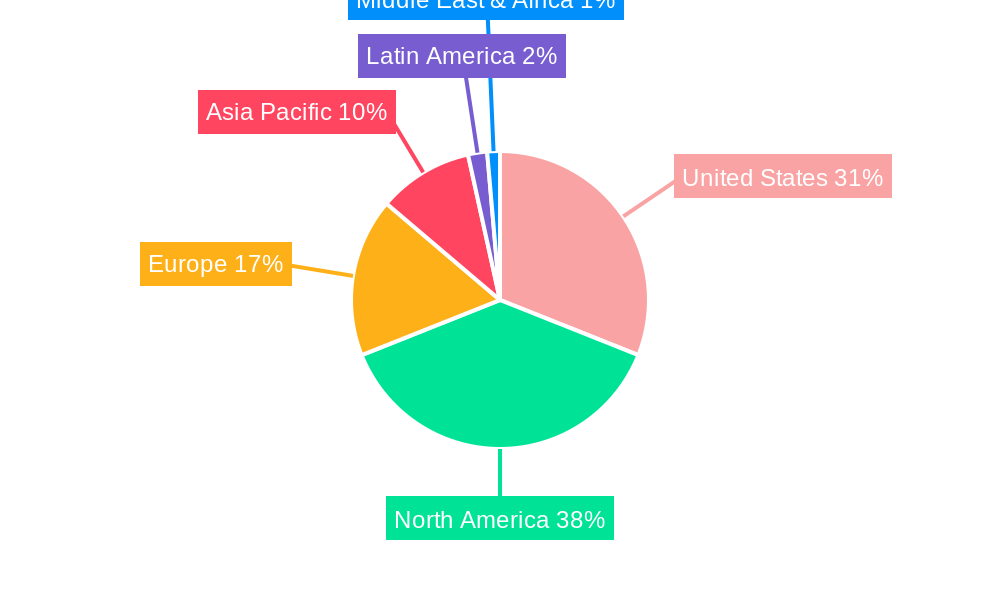
United States Neonatal and Prenatal Devices Market Regional Market Share

Geographic Coverage of United States Neonatal and Prenatal Devices Market
United States Neonatal and Prenatal Devices Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 6.1% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Rising Incidence of Preterm Births in the United States; Increasing Awareness for Prenatal and Neonatal Care; Government Initiatives to Provide Better Care for Prenatal and Neonatal Infants
- 3.3. Market Restrains
- 3.3.1. Risk of Nosocomial Infection from the Devices; Low Birth Rates
- 3.4. Market Trends
- 3.4.1. Neonatal Monitoring Devices are Expected to Witness Strong Growth Over the Forecast Period in Product Type Segment
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. United States Neonatal and Prenatal Devices Market Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Product Type
- 5.1.1. Prenatal and Fetal Equipment
- 5.1.1.1. Ultrasound and Ultrasonography Devices
- 5.1.1.2. Fetal Doppler
- 5.1.1.3. Fetal Magnetic Resonance Imaging (MRI)
- 5.1.1.4. Fetal Heart Monitors
- 5.1.1.5. Fetal Pulse Oximeters
- 5.1.1.6. Other Prenatal and Fetal Equipment
- 5.1.2. Neonatal Equipment
- 5.1.2.1. Incubators
- 5.1.2.2. Neonatal Monitoring Devices
- 5.1.2.3. Phototherapy Equipment
- 5.1.2.4. Respiratory Assistance and Monitoring Devices
- 5.1.2.5. Other Neonatal Care Equipment
- 5.1.1. Prenatal and Fetal Equipment
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. United States
- 5.1. Market Analysis, Insights and Forecast - by Product Type
- 6. Competitive Analysis
- 6.1. Market Share Analysis 2025
- 6.2. Company Profiles
- 6.2.1 Natus Medical Incorporated
- 6.2.1.1. Overview
- 6.2.1.2. Products
- 6.2.1.3. SWOT Analysis
- 6.2.1.4. Recent Developments
- 6.2.1.5. Financials (Based on Availability)
- 6.2.2 Phoenix Medical Systems (P) Ltd
- 6.2.2.1. Overview
- 6.2.2.2. Products
- 6.2.2.3. SWOT Analysis
- 6.2.2.4. Recent Developments
- 6.2.2.5. Financials (Based on Availability)
- 6.2.3 Masimo
- 6.2.3.1. Overview
- 6.2.3.2. Products
- 6.2.3.3. SWOT Analysis
- 6.2.3.4. Recent Developments
- 6.2.3.5. Financials (Based on Availability)
- 6.2.4 GE Healthcare
- 6.2.4.1. Overview
- 6.2.4.2. Products
- 6.2.4.3. SWOT Analysis
- 6.2.4.4. Recent Developments
- 6.2.4.5. Financials (Based on Availability)
- 6.2.5 Medtronic PLC
- 6.2.5.1. Overview
- 6.2.5.2. Products
- 6.2.5.3. SWOT Analysis
- 6.2.5.4. Recent Developments
- 6.2.5.5. Financials (Based on Availability)
- 6.2.6 Vyaire Medical
- 6.2.6.1. Overview
- 6.2.6.2. Products
- 6.2.6.3. SWOT Analysis
- 6.2.6.4. Recent Developments
- 6.2.6.5. Financials (Based on Availability)
- 6.2.7 Getinge AB
- 6.2.7.1. Overview
- 6.2.7.2. Products
- 6.2.7.3. SWOT Analysis
- 6.2.7.4. Recent Developments
- 6.2.7.5. Financials (Based on Availability)
- 6.2.8 Koninklijke Philips NV
- 6.2.8.1. Overview
- 6.2.8.2. Products
- 6.2.8.3. SWOT Analysis
- 6.2.8.4. Recent Developments
- 6.2.8.5. Financials (Based on Availability)
- 6.2.1 Natus Medical Incorporated
List of Figures
- Figure 1: United States Neonatal and Prenatal Devices Market Revenue Breakdown (billion, %) by Product 2025 & 2033
- Figure 2: United States Neonatal and Prenatal Devices Market Share (%) by Company 2025
List of Tables
- Table 1: United States Neonatal and Prenatal Devices Market Revenue billion Forecast, by Product Type 2020 & 2033
- Table 2: United States Neonatal and Prenatal Devices Market Volume K Units Forecast, by Product Type 2020 & 2033
- Table 3: United States Neonatal and Prenatal Devices Market Revenue billion Forecast, by Region 2020 & 2033
- Table 4: United States Neonatal and Prenatal Devices Market Volume K Units Forecast, by Region 2020 & 2033
- Table 5: United States Neonatal and Prenatal Devices Market Revenue billion Forecast, by Product Type 2020 & 2033
- Table 6: United States Neonatal and Prenatal Devices Market Volume K Units Forecast, by Product Type 2020 & 2033
- Table 7: United States Neonatal and Prenatal Devices Market Revenue billion Forecast, by Country 2020 & 2033
- Table 8: United States Neonatal and Prenatal Devices Market Volume K Units Forecast, by Country 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the United States Neonatal and Prenatal Devices Market?
The projected CAGR is approximately 6.1%.
2. Which companies are prominent players in the United States Neonatal and Prenatal Devices Market?
Key companies in the market include Natus Medical Incorporated, Phoenix Medical Systems (P) Ltd, Masimo, GE Healthcare, Medtronic PLC, Vyaire Medical, Getinge AB, Koninklijke Philips NV.
3. What are the main segments of the United States Neonatal and Prenatal Devices Market?
The market segments include Product Type.
4. Can you provide details about the market size?
The market size is estimated to be USD 4.8 billion as of 2022.
5. What are some drivers contributing to market growth?
Rising Incidence of Preterm Births in the United States; Increasing Awareness for Prenatal and Neonatal Care; Government Initiatives to Provide Better Care for Prenatal and Neonatal Infants.
6. What are the notable trends driving market growth?
Neonatal Monitoring Devices are Expected to Witness Strong Growth Over the Forecast Period in Product Type Segment.
7. Are there any restraints impacting market growth?
Risk of Nosocomial Infection from the Devices; Low Birth Rates.
8. Can you provide examples of recent developments in the market?
September 2022: Philips received FDA 510(k) clearance for the Ultrasound Compact system to optimize portability and performance.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in billion and volume, measured in K Units.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "United States Neonatal and Prenatal Devices Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the United States Neonatal and Prenatal Devices Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the United States Neonatal and Prenatal Devices Market?
To stay informed about further developments, trends, and reports in the United States Neonatal and Prenatal Devices Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
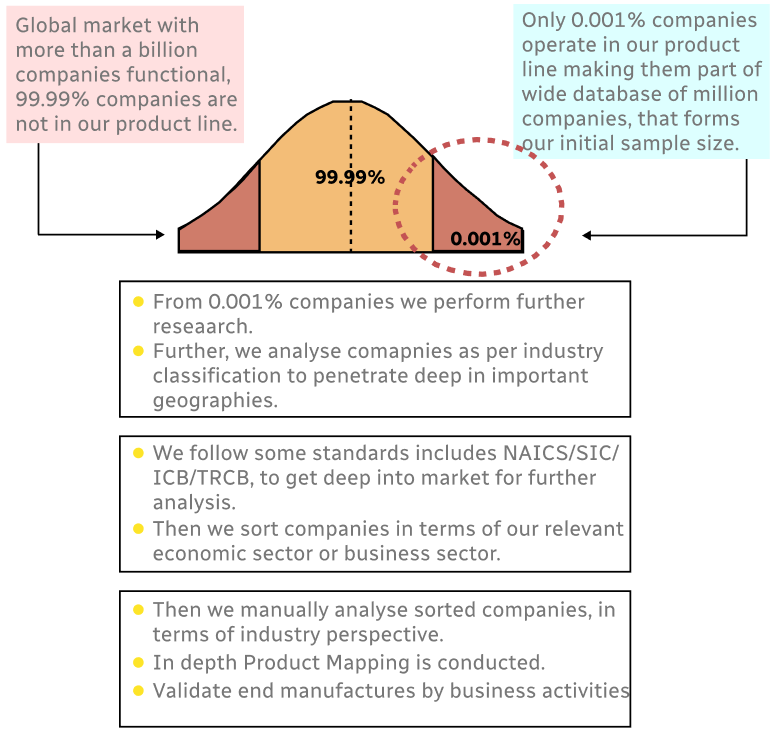
Step 1 - Identification of Relevant Samples Size from Population Database
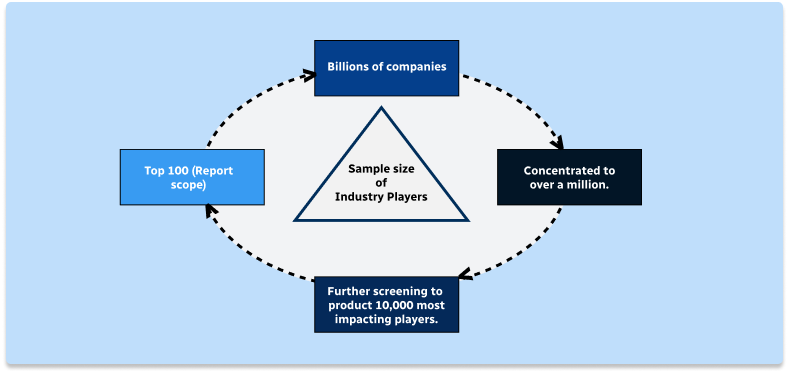
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
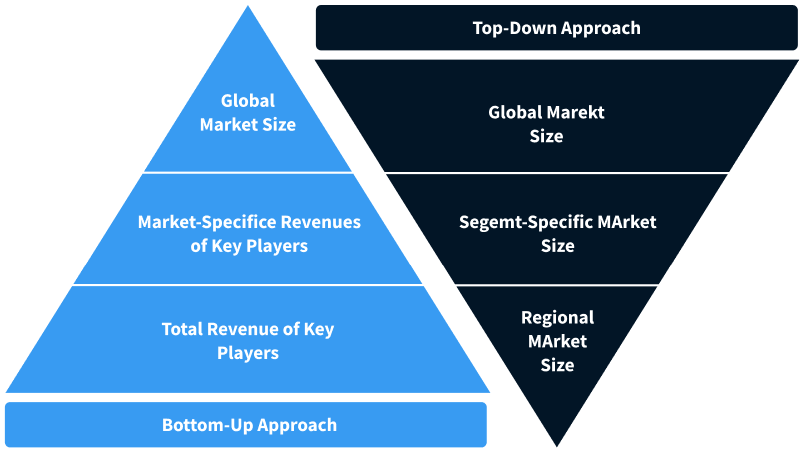
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
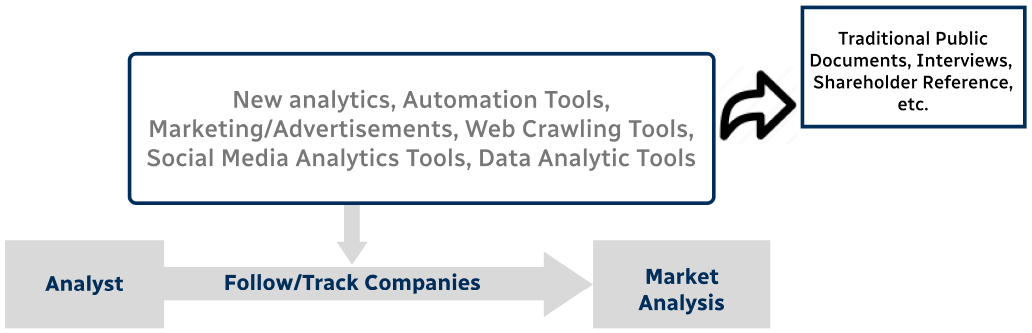
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


