Key Insights
The global Oseltamivir market is projected to reach USD 2490.8 million in 2024, exhibiting a CAGR of 1.1% over the forecast period of 2025-2033. This steady growth is underpinned by the persistent prevalence of influenza A and B viruses, which drive the demand for effective antiviral treatments. The market's expansion is further supported by an aging global population, which generally experiences a higher susceptibility to influenza and its complications. Additionally, increasing healthcare expenditure and enhanced diagnostic capabilities worldwide contribute to a more accurate and timely identification of influenza cases, thereby boosting the utilization of Oseltamivir. The availability of Oseltamivir in convenient dosage forms, such as capsules and suspensions, also plays a crucial role in its widespread adoption by both healthcare providers and patients.
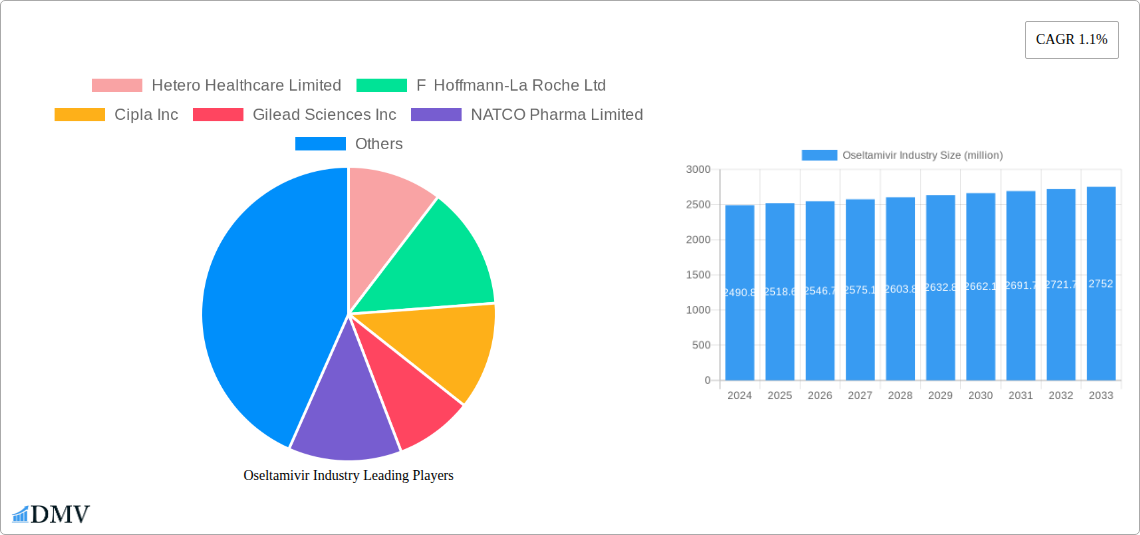
Oseltamivir Industry Market Size (In Billion)

Despite the generally stable market outlook, several factors influence its trajectory. Key drivers include the ongoing need for influenza prophylaxis and treatment, particularly during seasonal outbreaks and pandemic preparedness. Emerging trends such as the development of novel drug delivery systems and combination therapies aimed at improving efficacy and reducing resistance could also shape market dynamics. However, the market faces certain restraints, including the potential for drug resistance development in influenza viruses, which necessitates continuous research and development efforts. Stringent regulatory approvals for new formulations and the availability of alternative treatments, including vaccines and other antiviral agents, also present competitive challenges. The market's regional distribution is characterized by significant contributions from North America and Europe, owing to robust healthcare infrastructures and high public health awareness. Asia Pacific is expected to witness considerable growth due to its large population base and improving healthcare access.
Oseltamivir Industry Company Market Share

Oseltamivir Industry Market Composition & Trends
The Oseltamivir industry is characterized by a dynamic market composition, driven by the ongoing need for effective influenza treatment and prophylaxis. Market concentration is moderately high, with key players like F Hoffmann-La Roche Ltd, Cipla Inc, and Hetero Healthcare Limited holding significant market share. Innovation remains a critical catalyst, with ongoing research focused on improving drug delivery, efficacy against emerging strains, and combination therapies. Regulatory landscapes are robust, with stringent approval processes by bodies like the FDA and EMA ensuring product safety and efficacy. Substitute products, while present in the form of other antivirals and preventative measures, are often limited by specific indications, resistance profiles, or side effect concerns, solidifying Oseltamivir's position. End-user profiles span broad demographics, including individuals at high risk for influenza complications, healthcare institutions, and public health organizations. Mergers and acquisitions (M&A) activities, though not consistently high, have seen strategic consolidation to enhance manufacturing capabilities and market reach, with estimated deal values in the tens to hundreds of millions.
- Market Share Distribution: While specific figures fluctuate, major manufacturers collectively control over 70% of the global Oseltamivir market.
- M&A Deal Values: Recent strategic acquisitions and partnerships have ranged from approximately $50 million to $300 million, focused on expanding manufacturing capacity and market penetration.
- Innovation Catalysts: Development of new formulations, improved manufacturing processes, and clinical trials exploring Oseltamivir in novel therapeutic contexts.
- Regulatory Landscape: Strict adherence to cGMP standards, clinical trial protocols, and post-market surveillance.
- Substitute Products: Zanamivir, Peramivir, Baloxavir marboxil, and influenza vaccines represent key alternatives and complementary treatments.
Oseltamivir Industry Industry Evolution
The Oseltamivir industry has witnessed a significant evolution, marked by consistent growth trajectories, technological advancements, and shifting consumer demands over the historical period of 2019–2024 and projected through the forecast period of 2025–2033. The market's growth has been primarily propelled by the persistent threat of seasonal influenza outbreaks and the potential for pandemic influenza events. During the historical period, particularly with the heightened awareness and demand during the COVID-19 pandemic, the Oseltamivir market experienced an accelerated uptake. This was due to its established role in treating and preventing influenza, often recommended by healthcare professionals as a supportive measure or for individuals at high risk of severe outcomes. The base year of 2025 is anticipated to reflect a stable, yet growing market, as global health organizations continue to emphasize preparedness for influenza.
Technological advancements have played a crucial role in this evolution. Innovations in manufacturing processes have led to more efficient and cost-effective production of Oseltamivir, benefiting both branded and generic manufacturers. This includes improvements in active pharmaceutical ingredient (API) synthesis and formulation techniques, contributing to higher purity and better bioavailability. The development of generic versions has significantly increased market accessibility, especially in emerging economies, by driving down prices. The adoption of advanced quality control measures and streamlined supply chain management have also been integral to ensuring the availability of this essential antiviral medication.
Shifting consumer demands have also influenced the industry's trajectory. There's an increasing demand for accessible and affordable treatments, driving the market for generic Oseltamivir. Furthermore, growing awareness among the public and healthcare providers about the importance of early intervention for influenza symptoms, especially in vulnerable populations like the elderly, children, and immunocompromised individuals, has bolstered demand. The focus on preventative measures, including vaccination, has historically been a parallel trend, but the need for therapeutic interventions remains, creating a dual market dynamic. The development of oral suspensions has further catered to pediatric populations, expanding the user base. The strategic importance of maintaining robust stockpiles of Oseltamivir for pandemic preparedness by governments and health organizations continues to be a significant factor, ensuring a baseline level of demand and industry stability.
Leading Regions, Countries, or Segments in Oseltamivir Industry
The Oseltamivir industry demonstrates distinct regional dominance and segment leadership, driven by a confluence of factors including healthcare infrastructure, disease prevalence, regulatory policies, and economic development. North America, particularly the United States, has consistently emerged as a leading region in terms of market size and consumption. This leadership is underpinned by a highly developed healthcare system, robust public health initiatives focused on influenza surveillance and treatment, and a substantial demand for both prescription and over-the-counter (OTC) antiviral medications. The presence of major pharmaceutical players and a strong regulatory framework that encourages research and development further solidifies its position.
Within the Product Type segment, Capsule formulations of Oseltamivir hold a significant market share. This is attributed to their widespread availability, ease of administration for adult patients, and established prescription patterns by healthcare professionals. The convenience of a single dosage form for a significant portion of the patient population contributes to its sustained demand. However, the Suspension segment is experiencing robust growth, particularly driven by pediatric influenza treatment. The increasing recognition of the need for age-appropriate formulations and the proactive approach of pediatricians in prescribing oral suspensions for children have fueled this expansion.
In terms of Industry Vertical, Influenza A and Influenza B are the primary drivers for the Oseltamivir market. These are the most common strains responsible for seasonal epidemics, and Oseltamivir is a frontline treatment and prophylaxis for both. The cyclical nature of influenza seasons ensures a consistent demand, with peaks typically observed during colder months. Global health organizations and national health agencies heavily invest in surveillance and preparedness programs for both Influenza A and B, directly impacting the demand for effective antivirals like Oseltamivir.
- North America (USA & Canada): Dominant market due to advanced healthcare, strong public health programs, and significant R&D investment by key players.
- Europe (Germany, UK, France): Strong market presence driven by well-established healthcare systems and a high prevalence of seasonal influenza.
- Asia Pacific (China & India): Rapidly growing market due to increasing healthcare expenditure, large populations, and a growing generic pharmaceutical sector.
- Product Type - Capsule: Leading segment due to ease of administration for adult patients and established prescription patterns.
- Product Type - Suspension: High growth segment driven by pediatric influenza treatment and demand for age-appropriate formulations.
- Industry Vertical - Influenza A & B: Primary drivers of demand due to their prevalence in seasonal epidemics and the role of Oseltamivir in treatment and prophylaxis.
- Regulatory Support: Favorable regulatory environments in leading regions facilitate market access and product approvals.
- Investment Trends: Significant investments in influenza research and public health infrastructure contribute to regional dominance.
Oseltamivir Industry Product Innovations
Oseltamivir product innovations are primarily focused on enhancing patient convenience, improving therapeutic outcomes, and expanding its application scope. The development of novel oral formulations, such as extended-release capsules or more palatable suspension formulations for pediatric use, represents key advancements. Research into combination therapies, exploring Oseltamivir's synergistic effects with other antiviral agents or immunomodulators, is also a significant area of innovation, aiming to overcome drug resistance and improve efficacy against severe influenza infections. Performance metrics are continuously being refined through clinical trials evaluating its impact on reducing viral shedding, shortening illness duration, and preventing complications.
Propelling Factors for Oseltamivir Industry Growth
The Oseltamivir industry's growth is propelled by several critical factors, including the persistent threat of seasonal influenza outbreaks and the ever-present potential for pandemic events, necessitating robust antiviral stockpiles. Technological advancements in manufacturing have enhanced production efficiency and lowered costs, making generic versions more accessible and affordable. Furthermore, increasing global health awareness and government initiatives focused on influenza preparedness and response directly contribute to sustained demand. Favorable regulatory environments in key markets streamline product approvals and market entry, while ongoing research into new applications and combination therapies promises to expand its therapeutic utility.
Obstacles in the Oseltamivir Industry Market
Despite its established position, the Oseltamivir industry faces several obstacles. Regulatory hurdles, particularly for novel formulations or expanded indications, can be time-consuming and costly. Supply chain disruptions, exacerbated by global events or manufacturing challenges, can lead to intermittent shortages, impacting availability. Intense competition from generic manufacturers and the emergence of alternative antiviral treatments pose significant price pressures. Moreover, the development of influenza virus resistance to Oseltamivir, though currently manageable, remains a long-term concern that could necessitate shifts in treatment paradigms, potentially impacting market share.
Future Opportunities in Oseltamivir Industry
Future opportunities in the Oseltamivir industry lie in several promising avenues. The development of novel drug delivery systems, such as inhaled or injectable formulations, could offer alternative treatment options for specific patient groups or severe infections. Expanding Oseltamivir's use in prophylaxis for high-risk individuals during non-pandemic periods and exploring its role in managing other respiratory viral infections present significant growth potential. Furthermore, the increasing demand for antivirals in emerging economies, coupled with advancements in generic manufacturing, offers substantial market expansion opportunities. Collaborations with public health organizations for enhanced pandemic preparedness and rapid response stockpiling also represent a consistent revenue stream.
Major Players in the Oseltamivir Industry Ecosystem
- Hetero Healthcare Limited
- F Hoffmann-La Roche Ltd
- Cipla Inc
- Gilead Sciences Inc
- NATCO Pharma Limited
- Strides Pharma Science Limited
- Amneal Pharmaceuticals LLC
- Lupin Limited
- Alembic Pharmaceuticals Limited
- Macleods Pharmaceuticals Ltd
- Zydus Cadila
Key Developments in Oseltamivir Industry Industry
- October 2021: M.D. Anderson Cancer Center initiated a Phase II clinical study on the effect of baloxavir in combination with oseltamivir in treating severe influenza infection in patients who have previously received a hematopoietic (blood) stem cell transplant. This development signifies exploration into combination therapies for vulnerable populations.
- January 2022: Strides Pharma Science Ltd received approval from the U.S. health regulator for its generic version of oseltamivir phosphate for oral suspension, used for treating illness due to influenza. This milestone highlights the growing importance of accessible generic options and strengthens market competition.
Strategic Oseltamivir Industry Market Forecast
The Oseltamivir industry market forecast indicates continued steady growth, driven by its essential role in managing seasonal influenza and its strategic importance in global pandemic preparedness. The increasing prevalence of generic Oseltamivir, offering cost-effective treatment options, will fuel market expansion, particularly in emerging economies. Innovations in formulation and the ongoing exploration of combination therapies are expected to enhance its therapeutic efficacy and appeal. Furthermore, heightened awareness and proactive public health strategies surrounding influenza prevention and management will sustain demand. The forecast period from 2025 to 2033 anticipates a resilient market, capitalizing on its established efficacy and the ongoing need for reliable antiviral interventions.
Oseltamivir Industry Segmentation
-
1. Product Type
- 1.1. Capsule
- 1.2. Suspension
- 1.3. Other Product Types
-
2. Industry vertical
- 2.1. Influenza A
- 2.2. Influenza B
- 2.3. Other
Oseltamivir Industry Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. Europe
- 2.1. Germany
- 2.2. United Kingdom
- 2.3. France
- 2.4. Italy
- 2.5. Spain
- 2.6. Rest of Europe
-
3. Asia Pacific
- 3.1. China
- 3.2. Japan
- 3.3. India
- 3.4. Australia
- 3.5. South Korea
- 3.6. Rest of Asia Pacific
-
4. Middle East and Africa
- 4.1. GCC
- 4.2. South Africa
- 4.3. Rest of Middle East and Africa
-
5. South America
- 5.1. Brazil
- 5.2. Argentina
- 5.3. Rest of South America
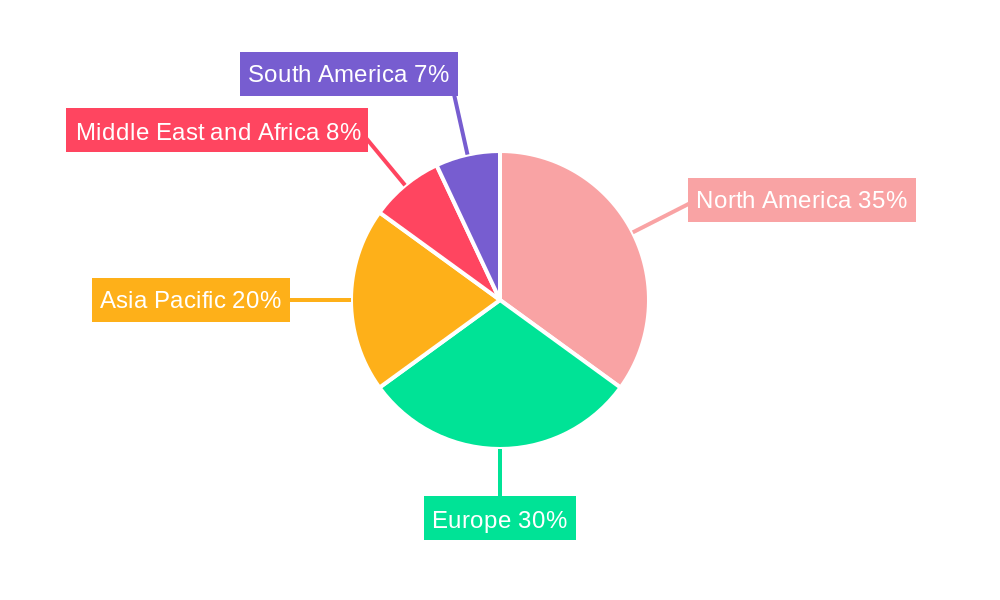
Oseltamivir Industry Regional Market Share

Geographic Coverage of Oseltamivir Industry
Oseltamivir Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2020-2034 |
| Base Year | 2025 |
| Estimated Year | 2026 |
| Forecast Period | 2026-2034 |
| Historical Period | 2020-2025 |
| Growth Rate | CAGR of 1.1% from 2020-2034 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Rising Prevalence of Influenza Virus Infections; Increase in the Number of Research and Development Activities
- 3.3. Market Restrains
- 3.3.1. Side Effects Associated with Oseltamivir Treatment
- 3.4. Market Trends
- 3.4.1. Influenza A Segment Expects to Register a High CAGR Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Oseltamivir Industry Analysis, Insights and Forecast, 2020-2032
- 5.1. Market Analysis, Insights and Forecast - by Product Type
- 5.1.1. Capsule
- 5.1.2. Suspension
- 5.1.3. Other Product Types
- 5.2. Market Analysis, Insights and Forecast - by Industry vertical
- 5.2.1. Influenza A
- 5.2.2. Influenza B
- 5.2.3. Other
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. North America
- 5.3.2. Europe
- 5.3.3. Asia Pacific
- 5.3.4. Middle East and Africa
- 5.3.5. South America
- 5.1. Market Analysis, Insights and Forecast - by Product Type
- 6. North America Oseltamivir Industry Analysis, Insights and Forecast, 2020-2032
- 6.1. Market Analysis, Insights and Forecast - by Product Type
- 6.1.1. Capsule
- 6.1.2. Suspension
- 6.1.3. Other Product Types
- 6.2. Market Analysis, Insights and Forecast - by Industry vertical
- 6.2.1. Influenza A
- 6.2.2. Influenza B
- 6.2.3. Other
- 6.1. Market Analysis, Insights and Forecast - by Product Type
- 7. Europe Oseltamivir Industry Analysis, Insights and Forecast, 2020-2032
- 7.1. Market Analysis, Insights and Forecast - by Product Type
- 7.1.1. Capsule
- 7.1.2. Suspension
- 7.1.3. Other Product Types
- 7.2. Market Analysis, Insights and Forecast - by Industry vertical
- 7.2.1. Influenza A
- 7.2.2. Influenza B
- 7.2.3. Other
- 7.1. Market Analysis, Insights and Forecast - by Product Type
- 8. Asia Pacific Oseltamivir Industry Analysis, Insights and Forecast, 2020-2032
- 8.1. Market Analysis, Insights and Forecast - by Product Type
- 8.1.1. Capsule
- 8.1.2. Suspension
- 8.1.3. Other Product Types
- 8.2. Market Analysis, Insights and Forecast - by Industry vertical
- 8.2.1. Influenza A
- 8.2.2. Influenza B
- 8.2.3. Other
- 8.1. Market Analysis, Insights and Forecast - by Product Type
- 9. Middle East and Africa Oseltamivir Industry Analysis, Insights and Forecast, 2020-2032
- 9.1. Market Analysis, Insights and Forecast - by Product Type
- 9.1.1. Capsule
- 9.1.2. Suspension
- 9.1.3. Other Product Types
- 9.2. Market Analysis, Insights and Forecast - by Industry vertical
- 9.2.1. Influenza A
- 9.2.2. Influenza B
- 9.2.3. Other
- 9.1. Market Analysis, Insights and Forecast - by Product Type
- 10. South America Oseltamivir Industry Analysis, Insights and Forecast, 2020-2032
- 10.1. Market Analysis, Insights and Forecast - by Product Type
- 10.1.1. Capsule
- 10.1.2. Suspension
- 10.1.3. Other Product Types
- 10.2. Market Analysis, Insights and Forecast - by Industry vertical
- 10.2.1. Influenza A
- 10.2.2. Influenza B
- 10.2.3. Other
- 10.1. Market Analysis, Insights and Forecast - by Product Type
- 11. Competitive Analysis
- 11.1. Global Market Share Analysis 2025
- 11.2. Company Profiles
- 11.2.1 Hetero Healthcare Limited
- 11.2.1.1. Overview
- 11.2.1.2. Products
- 11.2.1.3. SWOT Analysis
- 11.2.1.4. Recent Developments
- 11.2.1.5. Financials (Based on Availability)
- 11.2.2 F Hoffmann-La Roche Ltd
- 11.2.2.1. Overview
- 11.2.2.2. Products
- 11.2.2.3. SWOT Analysis
- 11.2.2.4. Recent Developments
- 11.2.2.5. Financials (Based on Availability)
- 11.2.3 Cipla Inc
- 11.2.3.1. Overview
- 11.2.3.2. Products
- 11.2.3.3. SWOT Analysis
- 11.2.3.4. Recent Developments
- 11.2.3.5. Financials (Based on Availability)
- 11.2.4 Gilead Sciences Inc
- 11.2.4.1. Overview
- 11.2.4.2. Products
- 11.2.4.3. SWOT Analysis
- 11.2.4.4. Recent Developments
- 11.2.4.5. Financials (Based on Availability)
- 11.2.5 NATCO Pharma Limited
- 11.2.5.1. Overview
- 11.2.5.2. Products
- 11.2.5.3. SWOT Analysis
- 11.2.5.4. Recent Developments
- 11.2.5.5. Financials (Based on Availability)
- 11.2.6 Strides Pharma Science Limited
- 11.2.6.1. Overview
- 11.2.6.2. Products
- 11.2.6.3. SWOT Analysis
- 11.2.6.4. Recent Developments
- 11.2.6.5. Financials (Based on Availability)
- 11.2.7 Amneal Pharmaceuticals LLC
- 11.2.7.1. Overview
- 11.2.7.2. Products
- 11.2.7.3. SWOT Analysis
- 11.2.7.4. Recent Developments
- 11.2.7.5. Financials (Based on Availability)
- 11.2.8 Lupin Limited
- 11.2.8.1. Overview
- 11.2.8.2. Products
- 11.2.8.3. SWOT Analysis
- 11.2.8.4. Recent Developments
- 11.2.8.5. Financials (Based on Availability)
- 11.2.9 Alembic Pharmaceuticals Limited
- 11.2.9.1. Overview
- 11.2.9.2. Products
- 11.2.9.3. SWOT Analysis
- 11.2.9.4. Recent Developments
- 11.2.9.5. Financials (Based on Availability)
- 11.2.10 Macleods Pharmaceuticals Ltd
- 11.2.10.1. Overview
- 11.2.10.2. Products
- 11.2.10.3. SWOT Analysis
- 11.2.10.4. Recent Developments
- 11.2.10.5. Financials (Based on Availability)
- 11.2.11 Zydus Cadila
- 11.2.11.1. Overview
- 11.2.11.2. Products
- 11.2.11.3. SWOT Analysis
- 11.2.11.4. Recent Developments
- 11.2.11.5. Financials (Based on Availability)
- 11.2.1 Hetero Healthcare Limited
List of Figures
- Figure 1: Global Oseltamivir Industry Revenue Breakdown (million, %) by Region 2025 & 2033
- Figure 2: Global Oseltamivir Industry Volume Breakdown (K Unit, %) by Region 2025 & 2033
- Figure 3: North America Oseltamivir Industry Revenue (million), by Product Type 2025 & 2033
- Figure 4: North America Oseltamivir Industry Volume (K Unit), by Product Type 2025 & 2033
- Figure 5: North America Oseltamivir Industry Revenue Share (%), by Product Type 2025 & 2033
- Figure 6: North America Oseltamivir Industry Volume Share (%), by Product Type 2025 & 2033
- Figure 7: North America Oseltamivir Industry Revenue (million), by Industry vertical 2025 & 2033
- Figure 8: North America Oseltamivir Industry Volume (K Unit), by Industry vertical 2025 & 2033
- Figure 9: North America Oseltamivir Industry Revenue Share (%), by Industry vertical 2025 & 2033
- Figure 10: North America Oseltamivir Industry Volume Share (%), by Industry vertical 2025 & 2033
- Figure 11: North America Oseltamivir Industry Revenue (million), by Country 2025 & 2033
- Figure 12: North America Oseltamivir Industry Volume (K Unit), by Country 2025 & 2033
- Figure 13: North America Oseltamivir Industry Revenue Share (%), by Country 2025 & 2033
- Figure 14: North America Oseltamivir Industry Volume Share (%), by Country 2025 & 2033
- Figure 15: Europe Oseltamivir Industry Revenue (million), by Product Type 2025 & 2033
- Figure 16: Europe Oseltamivir Industry Volume (K Unit), by Product Type 2025 & 2033
- Figure 17: Europe Oseltamivir Industry Revenue Share (%), by Product Type 2025 & 2033
- Figure 18: Europe Oseltamivir Industry Volume Share (%), by Product Type 2025 & 2033
- Figure 19: Europe Oseltamivir Industry Revenue (million), by Industry vertical 2025 & 2033
- Figure 20: Europe Oseltamivir Industry Volume (K Unit), by Industry vertical 2025 & 2033
- Figure 21: Europe Oseltamivir Industry Revenue Share (%), by Industry vertical 2025 & 2033
- Figure 22: Europe Oseltamivir Industry Volume Share (%), by Industry vertical 2025 & 2033
- Figure 23: Europe Oseltamivir Industry Revenue (million), by Country 2025 & 2033
- Figure 24: Europe Oseltamivir Industry Volume (K Unit), by Country 2025 & 2033
- Figure 25: Europe Oseltamivir Industry Revenue Share (%), by Country 2025 & 2033
- Figure 26: Europe Oseltamivir Industry Volume Share (%), by Country 2025 & 2033
- Figure 27: Asia Pacific Oseltamivir Industry Revenue (million), by Product Type 2025 & 2033
- Figure 28: Asia Pacific Oseltamivir Industry Volume (K Unit), by Product Type 2025 & 2033
- Figure 29: Asia Pacific Oseltamivir Industry Revenue Share (%), by Product Type 2025 & 2033
- Figure 30: Asia Pacific Oseltamivir Industry Volume Share (%), by Product Type 2025 & 2033
- Figure 31: Asia Pacific Oseltamivir Industry Revenue (million), by Industry vertical 2025 & 2033
- Figure 32: Asia Pacific Oseltamivir Industry Volume (K Unit), by Industry vertical 2025 & 2033
- Figure 33: Asia Pacific Oseltamivir Industry Revenue Share (%), by Industry vertical 2025 & 2033
- Figure 34: Asia Pacific Oseltamivir Industry Volume Share (%), by Industry vertical 2025 & 2033
- Figure 35: Asia Pacific Oseltamivir Industry Revenue (million), by Country 2025 & 2033
- Figure 36: Asia Pacific Oseltamivir Industry Volume (K Unit), by Country 2025 & 2033
- Figure 37: Asia Pacific Oseltamivir Industry Revenue Share (%), by Country 2025 & 2033
- Figure 38: Asia Pacific Oseltamivir Industry Volume Share (%), by Country 2025 & 2033
- Figure 39: Middle East and Africa Oseltamivir Industry Revenue (million), by Product Type 2025 & 2033
- Figure 40: Middle East and Africa Oseltamivir Industry Volume (K Unit), by Product Type 2025 & 2033
- Figure 41: Middle East and Africa Oseltamivir Industry Revenue Share (%), by Product Type 2025 & 2033
- Figure 42: Middle East and Africa Oseltamivir Industry Volume Share (%), by Product Type 2025 & 2033
- Figure 43: Middle East and Africa Oseltamivir Industry Revenue (million), by Industry vertical 2025 & 2033
- Figure 44: Middle East and Africa Oseltamivir Industry Volume (K Unit), by Industry vertical 2025 & 2033
- Figure 45: Middle East and Africa Oseltamivir Industry Revenue Share (%), by Industry vertical 2025 & 2033
- Figure 46: Middle East and Africa Oseltamivir Industry Volume Share (%), by Industry vertical 2025 & 2033
- Figure 47: Middle East and Africa Oseltamivir Industry Revenue (million), by Country 2025 & 2033
- Figure 48: Middle East and Africa Oseltamivir Industry Volume (K Unit), by Country 2025 & 2033
- Figure 49: Middle East and Africa Oseltamivir Industry Revenue Share (%), by Country 2025 & 2033
- Figure 50: Middle East and Africa Oseltamivir Industry Volume Share (%), by Country 2025 & 2033
- Figure 51: South America Oseltamivir Industry Revenue (million), by Product Type 2025 & 2033
- Figure 52: South America Oseltamivir Industry Volume (K Unit), by Product Type 2025 & 2033
- Figure 53: South America Oseltamivir Industry Revenue Share (%), by Product Type 2025 & 2033
- Figure 54: South America Oseltamivir Industry Volume Share (%), by Product Type 2025 & 2033
- Figure 55: South America Oseltamivir Industry Revenue (million), by Industry vertical 2025 & 2033
- Figure 56: South America Oseltamivir Industry Volume (K Unit), by Industry vertical 2025 & 2033
- Figure 57: South America Oseltamivir Industry Revenue Share (%), by Industry vertical 2025 & 2033
- Figure 58: South America Oseltamivir Industry Volume Share (%), by Industry vertical 2025 & 2033
- Figure 59: South America Oseltamivir Industry Revenue (million), by Country 2025 & 2033
- Figure 60: South America Oseltamivir Industry Volume (K Unit), by Country 2025 & 2033
- Figure 61: South America Oseltamivir Industry Revenue Share (%), by Country 2025 & 2033
- Figure 62: South America Oseltamivir Industry Volume Share (%), by Country 2025 & 2033
List of Tables
- Table 1: Global Oseltamivir Industry Revenue million Forecast, by Product Type 2020 & 2033
- Table 2: Global Oseltamivir Industry Volume K Unit Forecast, by Product Type 2020 & 2033
- Table 3: Global Oseltamivir Industry Revenue million Forecast, by Industry vertical 2020 & 2033
- Table 4: Global Oseltamivir Industry Volume K Unit Forecast, by Industry vertical 2020 & 2033
- Table 5: Global Oseltamivir Industry Revenue million Forecast, by Region 2020 & 2033
- Table 6: Global Oseltamivir Industry Volume K Unit Forecast, by Region 2020 & 2033
- Table 7: Global Oseltamivir Industry Revenue million Forecast, by Product Type 2020 & 2033
- Table 8: Global Oseltamivir Industry Volume K Unit Forecast, by Product Type 2020 & 2033
- Table 9: Global Oseltamivir Industry Revenue million Forecast, by Industry vertical 2020 & 2033
- Table 10: Global Oseltamivir Industry Volume K Unit Forecast, by Industry vertical 2020 & 2033
- Table 11: Global Oseltamivir Industry Revenue million Forecast, by Country 2020 & 2033
- Table 12: Global Oseltamivir Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 13: United States Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 14: United States Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 15: Canada Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 16: Canada Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 17: Mexico Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 18: Mexico Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 19: Global Oseltamivir Industry Revenue million Forecast, by Product Type 2020 & 2033
- Table 20: Global Oseltamivir Industry Volume K Unit Forecast, by Product Type 2020 & 2033
- Table 21: Global Oseltamivir Industry Revenue million Forecast, by Industry vertical 2020 & 2033
- Table 22: Global Oseltamivir Industry Volume K Unit Forecast, by Industry vertical 2020 & 2033
- Table 23: Global Oseltamivir Industry Revenue million Forecast, by Country 2020 & 2033
- Table 24: Global Oseltamivir Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 25: Germany Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 26: Germany Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 27: United Kingdom Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 28: United Kingdom Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 29: France Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 30: France Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 31: Italy Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 32: Italy Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 33: Spain Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 34: Spain Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 35: Rest of Europe Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 36: Rest of Europe Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 37: Global Oseltamivir Industry Revenue million Forecast, by Product Type 2020 & 2033
- Table 38: Global Oseltamivir Industry Volume K Unit Forecast, by Product Type 2020 & 2033
- Table 39: Global Oseltamivir Industry Revenue million Forecast, by Industry vertical 2020 & 2033
- Table 40: Global Oseltamivir Industry Volume K Unit Forecast, by Industry vertical 2020 & 2033
- Table 41: Global Oseltamivir Industry Revenue million Forecast, by Country 2020 & 2033
- Table 42: Global Oseltamivir Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 43: China Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 44: China Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 45: Japan Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 46: Japan Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 47: India Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 48: India Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 49: Australia Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 50: Australia Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 51: South Korea Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 52: South Korea Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 53: Rest of Asia Pacific Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 54: Rest of Asia Pacific Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 55: Global Oseltamivir Industry Revenue million Forecast, by Product Type 2020 & 2033
- Table 56: Global Oseltamivir Industry Volume K Unit Forecast, by Product Type 2020 & 2033
- Table 57: Global Oseltamivir Industry Revenue million Forecast, by Industry vertical 2020 & 2033
- Table 58: Global Oseltamivir Industry Volume K Unit Forecast, by Industry vertical 2020 & 2033
- Table 59: Global Oseltamivir Industry Revenue million Forecast, by Country 2020 & 2033
- Table 60: Global Oseltamivir Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 61: GCC Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 62: GCC Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 63: South Africa Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 64: South Africa Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 65: Rest of Middle East and Africa Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 66: Rest of Middle East and Africa Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 67: Global Oseltamivir Industry Revenue million Forecast, by Product Type 2020 & 2033
- Table 68: Global Oseltamivir Industry Volume K Unit Forecast, by Product Type 2020 & 2033
- Table 69: Global Oseltamivir Industry Revenue million Forecast, by Industry vertical 2020 & 2033
- Table 70: Global Oseltamivir Industry Volume K Unit Forecast, by Industry vertical 2020 & 2033
- Table 71: Global Oseltamivir Industry Revenue million Forecast, by Country 2020 & 2033
- Table 72: Global Oseltamivir Industry Volume K Unit Forecast, by Country 2020 & 2033
- Table 73: Brazil Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 74: Brazil Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 75: Argentina Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 76: Argentina Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
- Table 77: Rest of South America Oseltamivir Industry Revenue (million) Forecast, by Application 2020 & 2033
- Table 78: Rest of South America Oseltamivir Industry Volume (K Unit) Forecast, by Application 2020 & 2033
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Oseltamivir Industry?
The projected CAGR is approximately 1.1%.
2. Which companies are prominent players in the Oseltamivir Industry?
Key companies in the market include Hetero Healthcare Limited, F Hoffmann-La Roche Ltd, Cipla Inc, Gilead Sciences Inc, NATCO Pharma Limited, Strides Pharma Science Limited, Amneal Pharmaceuticals LLC, Lupin Limited, Alembic Pharmaceuticals Limited, Macleods Pharmaceuticals Ltd, Zydus Cadila.
3. What are the main segments of the Oseltamivir Industry?
The market segments include Product Type, Industry vertical.
4. Can you provide details about the market size?
The market size is estimated to be USD 2490.8 million as of 2022.
5. What are some drivers contributing to market growth?
Rising Prevalence of Influenza Virus Infections; Increase in the Number of Research and Development Activities.
6. What are the notable trends driving market growth?
Influenza A Segment Expects to Register a High CAGR Over the Forecast Period.
7. Are there any restraints impacting market growth?
Side Effects Associated with Oseltamivir Treatment.
8. Can you provide examples of recent developments in the market?
October 2021: M.D. Anderson Cancer Center initiated a Phase II clinical study on the effect of baloxavir in combination with oseltamivir in treating severe influenza infection in patients who have previously received a hematopoietic (blood) stem cell transplant.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Oseltamivir Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Oseltamivir Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Oseltamivir Industry?
To stay informed about further developments, trends, and reports in the Oseltamivir Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
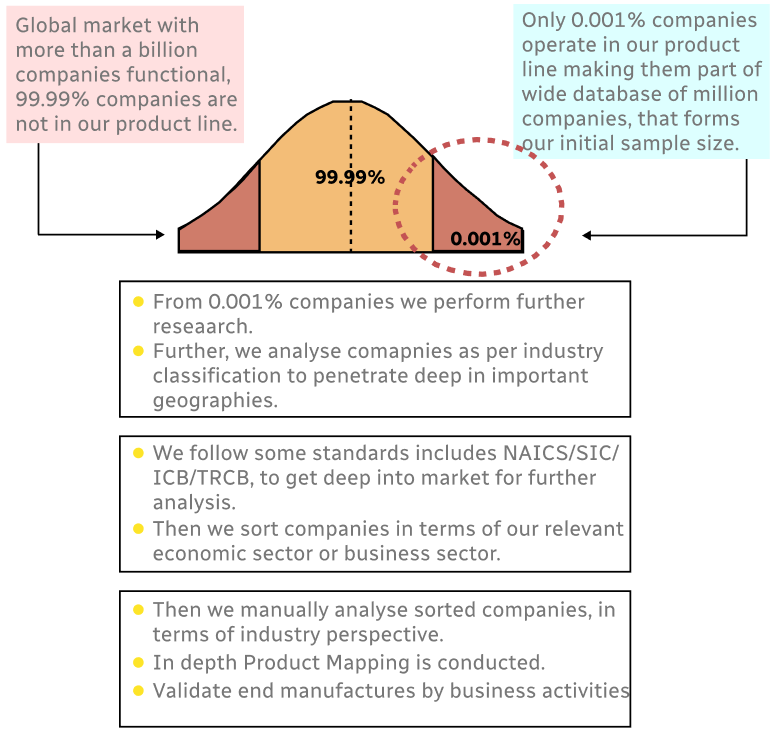
Step 1 - Identification of Relevant Samples Size from Population Database
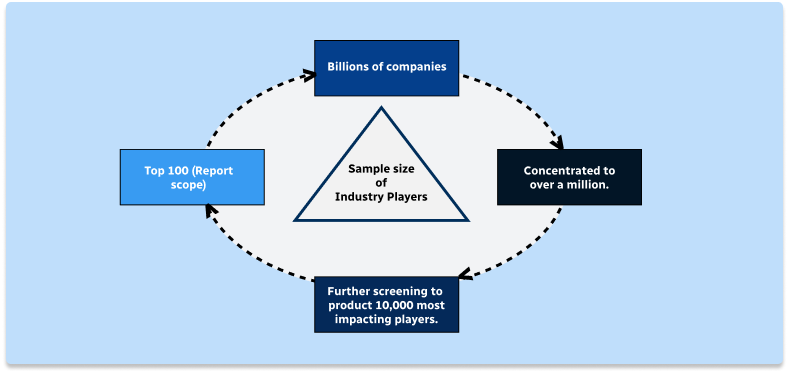
Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)
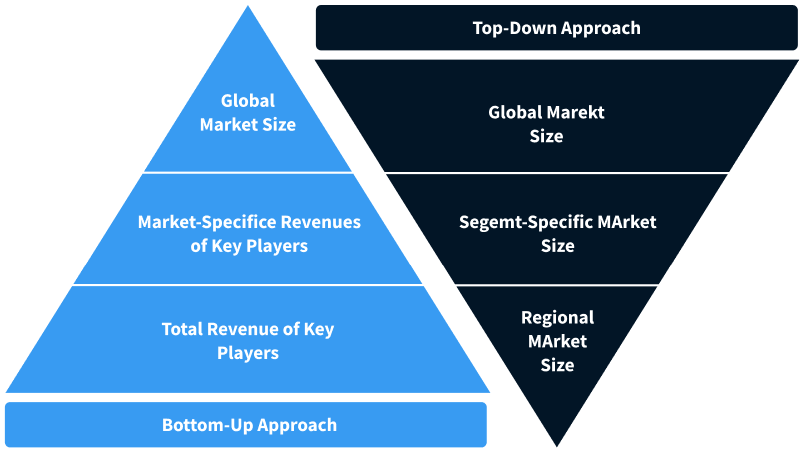
Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations
Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


