Key Insights
The European spinal surgery devices market is experiencing robust growth, projected to reach a substantial size within the forecast period of 2025-2033. A compound annual growth rate (CAGR) of 6.30% indicates a consistently expanding market driven by several key factors. The aging population across Europe is leading to an increased prevalence of spinal disorders requiring surgical intervention. Technological advancements in minimally invasive procedures, such as spinal decompression and fusion techniques using innovative materials like PEEK and titanium, are contributing to improved patient outcomes and driving market expansion. Furthermore, the rising adoption of advanced imaging technologies for accurate diagnosis and improved surgical planning fuels market growth. The market is segmented by device type (spinal decompression, spinal fusion, arthroplasty, non-fusion procedures), material (titanium, PEEK, ceramic), and end-user (hospitals, clinics, orthopedic centers). Germany, the United Kingdom, France, Italy, and Spain represent key regional markets within Europe, each contributing significantly to overall market value. While market restraints such as high procedure costs and the potential for complications associated with spinal surgery exist, the overall growth trajectory remains positive, driven by the aforementioned factors.
Growth within specific segments is expected to vary. For instance, the demand for minimally invasive spinal surgery devices is likely to outpace traditional procedures, driven by the preference for reduced recovery times and smaller incisions. Similarly, the use of advanced biocompatible materials like PEEK is anticipated to increase, owing to its superior strength and biocompatibility compared to traditional materials. The competitive landscape includes established players like Zimmer Biomet, Medtronic, and Johnson & Johnson (DePuy Synthes), alongside several other specialized companies, indicating a dynamic and innovative market environment. The consistent CAGR, alongside market drivers, points towards sustained growth for the European spinal surgery devices market over the coming years. The substantial investment in research and development by key market players further solidifies the potential for the continuous introduction of innovative products and procedures, sustaining this positive market outlook.
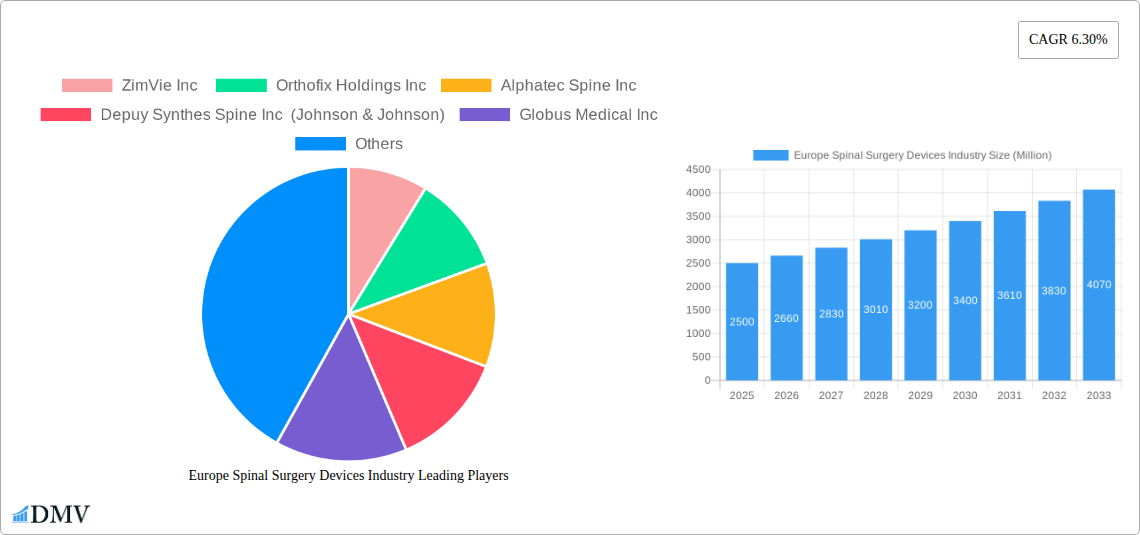
Europe Spinal Surgery Devices Industry: A Comprehensive Market Report (2019-2033)
This insightful report provides a detailed analysis of the Europe spinal surgery devices market, encompassing historical data (2019-2024), the base year (2025), and a comprehensive forecast (2025-2033). It offers crucial insights for stakeholders, investors, and industry professionals seeking to understand the market's dynamics, growth potential, and key players. The report covers a market valued at xx Million in 2025, projected to reach xx Million by 2033, exhibiting a CAGR of xx%.
Europe Spinal Surgery Devices Industry Market Composition & Trends
This section delves into the competitive landscape of the European spinal surgery devices market, examining market concentration, innovation drivers, regulatory frameworks, substitute products, end-user profiles, and merger & acquisition (M&A) activity. We analyze market share distribution among key players, revealing the dominance of a few large corporations while also highlighting the presence of smaller, innovative companies. The report includes analysis of M&A deal values, providing context to the consolidation trend within the industry.
- Market Concentration: The European spinal surgery devices market demonstrates a moderately concentrated structure, with the top five players holding an estimated xx% market share in 2025. This concentration is expected to slightly increase by 2033 due to ongoing M&A activity.
- Innovation Catalysts: Technological advancements, particularly in minimally invasive surgery (MIS) techniques and biomaterials, are major drivers of innovation. The increasing demand for improved patient outcomes and shorter recovery times fuels the development of advanced spinal implants and instrumentation.
- Regulatory Landscape: The stringent regulatory environment in Europe, governed by bodies like the European Medicines Agency (EMA), significantly impacts market access and product approvals. Compliance with these regulations is a key challenge for companies operating in this market.
- Substitute Products: While surgical intervention remains the primary treatment for many spinal conditions, alternative therapies like physiotherapy and medication exert a degree of competitive pressure. However, the severity and complexity of many spinal pathologies limit the effectiveness of these substitute options.
- End-User Profile: The primary end-users include hospitals, specialized spinal clinics, and orthopedic centers. The report analyzes the distribution of devices across these end-user segments, considering factors like hospital bed capacity and regional healthcare expenditure.
- M&A Activity: The European spinal surgery devices market has witnessed significant M&A activity in recent years, driven by companies seeking to expand their product portfolios, gain market share, and access new technologies. The report includes a detailed analysis of major deals, including deal values and strategic rationales. For example, the acquisition of Spine Innovations by Spineway in July 2022 expanded Spineway’s offerings in cervical and lumbar disc prostheses. The total value of M&A deals in the period 2019-2024 is estimated at xx Million.
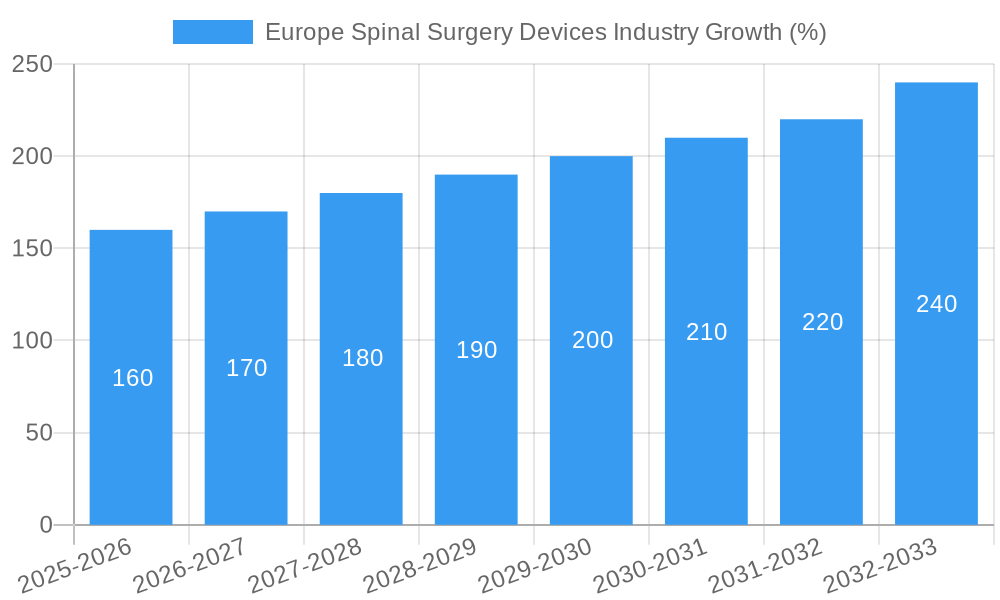
Europe Spinal Surgery Devices Industry Industry Evolution
This section examines the evolution of the European spinal surgery devices market, analyzing market growth trajectories, technological advancements, and shifting consumer demands. The analysis considers the historical period (2019-2024) and projects future trends based on identified growth drivers and market dynamics. We provide specific data points, including growth rates and adoption metrics for various device types and materials. Factors such as increasing prevalence of spinal disorders, an aging population, and growing awareness about minimally invasive surgical options are identified as key factors driving market expansion. Technological advancements like 3D-printing and robotic-assisted surgery are expected to reshape the market landscape in the forecast period (2025-2033). The increasing focus on value-based healthcare and cost-effectiveness will also influence device adoption and market dynamics. The report further analyzes the impact of changing reimbursement policies and healthcare regulations on market growth. Specific growth rate data for the historical and forecast periods are included, along with detailed market segmentation based on device type, material, and end-user. For example, the market segment of Spinal Fusion is expected to witness a growth rate of xx% from 2025 to 2033.
Leading Regions, Countries, or Segments in Europe Spinal Surgery Devices Industry
This section identifies the leading regions, countries, and segments within the European spinal surgery devices market. We analyze the key drivers behind the dominance of specific regions and segments, including investment trends, regulatory support, healthcare infrastructure, and prevalence of spinal disorders.
Dominant Regions: Germany, France, and the United Kingdom are expected to remain the leading markets in Europe due to factors including high healthcare expenditure, advanced medical infrastructure, and a relatively high prevalence of spinal pathologies.
Dominant Countries: [Detailed analysis of specific country performance will be provided here, explaining factors like market size, growth rate, and regulatory frameworks. This analysis will incorporate data for Germany, France, UK, Italy, Spain, etc. ]
Dominant Segments:
- Device Type: Spinal fusion devices are currently the largest segment, driven by the high prevalence of degenerative disc disease and spinal trauma. However, the non-fusion segment is projected to experience faster growth due to the increasing adoption of less invasive surgical techniques.
- Material: Titanium remains the most widely used material due to its biocompatibility and strength. However, PEEK is gaining traction due to its superior mechanical properties and radiolucency.
- End-User: Hospitals represent the largest segment of end-users, followed by specialized spinal clinics and orthopedic centers.
Europe Spinal Surgery Devices Industry Product Innovations
Recent product innovations have focused on improving surgical precision, minimizing invasiveness, and enhancing patient outcomes. Advancements in biomaterials, such as the development of porous titanium implants for enhanced bone integration, and the integration of advanced imaging technologies into surgical instruments, are significant examples. Companies are also focusing on developing minimally invasive surgical (MIS) devices that reduce trauma, shorten recovery times, and improve patient satisfaction. The incorporation of smart technologies, like sensors for real-time monitoring of implant stability, is another emerging trend contributing to improved patient care.
Propelling Factors for Europe Spinal Surgery Devices Industry Growth
The growth of the European spinal surgery devices market is propelled by a confluence of factors: the rising prevalence of age-related spinal disorders in an aging population, technological advancements in minimally invasive surgical techniques and biomaterials, increasing healthcare expenditure across Europe, and supportive regulatory frameworks encouraging innovation. Furthermore, the growing awareness among patients about advanced treatment options and improved surgical outcomes further contributes to market expansion.
Obstacles in the Europe Spinal Surgery Devices Industry Market
Despite the positive growth outlook, several challenges hinder market expansion. Stringent regulatory approvals and reimbursement policies increase the time and cost associated with product launches. Supply chain disruptions can impact the availability of raw materials and finished products. Intense competition among established players and the emergence of new entrants creates pricing pressure and necessitates continuous innovation to maintain market share. The overall impact of these challenges results in an estimated xx% reduction in market growth potential.
Future Opportunities in Europe Spinal Surgery Devices Industry
Future opportunities reside in the development of innovative technologies such as personalized implants, advanced imaging guidance systems, and smart implants with integrated sensors for improved patient monitoring. Further growth is anticipated from the expansion into emerging markets within Europe, coupled with increased investment in research and development. The rising demand for minimally invasive surgeries and focus on value-based healthcare will also open up opportunities for companies offering cost-effective and efficient solutions.
Major Players in the Europe Spinal Surgery Devices Industry Ecosystem
- ZimVie Inc
- Orthofix Holdings Inc
- Alphatec Spine Inc
- Depuy Synthes Spine Inc (Johnson & Johnson)
- Globus Medical Inc
- Medtronic PLC
- SeaSpine Inc
- SpineGuard SA
- Stryker Corporation
- NuVasive Inc
Key Developments in Europe Spinal Surgery Devices Industry Industry
- July 2022: Spineway acquired 100% of Spine Innovations, strengthening its position in cervical and lumbar disc prostheses. This acquisition significantly broadened Spineway's product portfolio and market reach.
- June 2022: NGMedical GmbH launched its ART Fixation System, introducing a new innovative spinal fixation technology into the European market. This launch intensified competition and provided surgeons with an additional treatment option.
Strategic Europe Spinal Surgery Devices Industry Market Forecast
The European spinal surgery devices market is poised for sustained growth driven by the confluence of factors previously discussed. The increasing prevalence of spinal disorders, combined with technological advancements and a growing emphasis on minimally invasive procedures, will create significant opportunities for market expansion. The forecast period (2025-2033) is expected to witness substantial growth, particularly in segments focused on minimally invasive technologies and advanced biomaterials. The overall market potential is considerable, driven by unmet clinical needs, an aging population, and continuing innovation in the industry.
Europe Spinal Surgery Devices Industry Segmentation
-
1. Device Type
-
1.1. Spinal Decompression
- 1.1.1. Corpectomy
- 1.1.2. Discectomy
- 1.1.3. Facetectomy
- 1.1.4. Foraminotomy
- 1.1.5. Laminotomy
-
1.2. Spinal Fusion
- 1.2.1. Cervical Fusion
- 1.2.2. Interbody Fusion
- 1.2.3. Thoraco Lumbar Fusion
- 1.2.4. Other Spinal Fusions
- 1.3. Fracture Repair
- 1.4. Arthroplasty
- 1.5. Non-fusion Procedures
-
1.1. Spinal Decompression
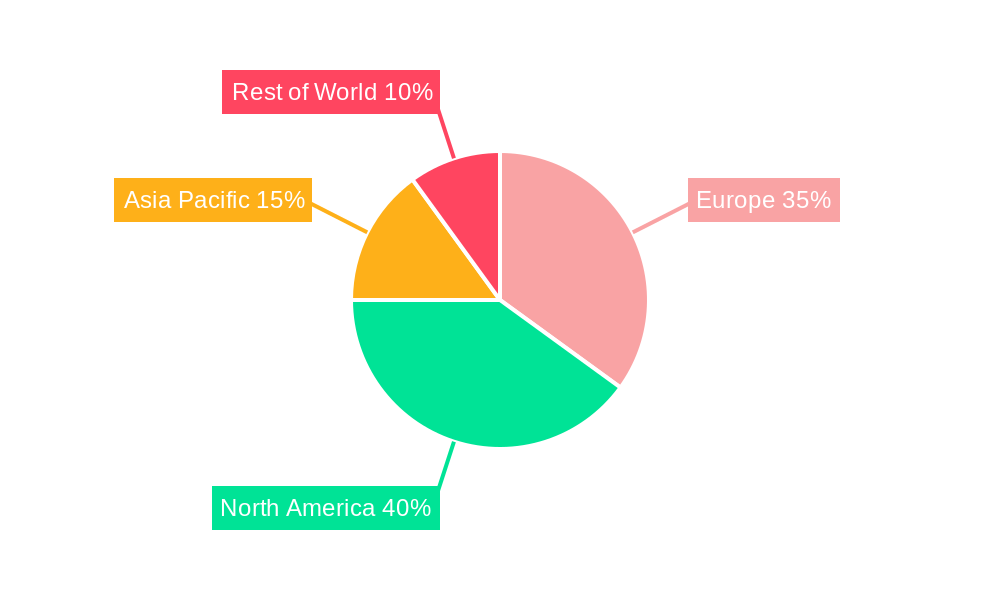
Europe Spinal Surgery Devices Industry Segmentation By Geography
- 1. Germany
- 2. United Kingdom
- 3. France
- 4. Italy
- 5. Spain
- 6. Rest of Europe
Europe Spinal Surgery Devices Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 6.30% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1 Increasing Demand for Minimally Invasive Surgical Procedures; Increasing Incidence of Obesity
- 3.2.2 Aging Population
- 3.2.3 and Associated Spine Disorders; Continuous Advancements in Spine Surgery Technologies
- 3.3. Market Restrains
- 3.3.1. High Cost and Time Involved in Treatment Procedures; Reimbursement Issues
- 3.4. Market Trends
- 3.4.1. Lumbar Fusion is Expected to Witness a Significant Growth Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 5.1.1. Spinal Decompression
- 5.1.1.1. Corpectomy
- 5.1.1.2. Discectomy
- 5.1.1.3. Facetectomy
- 5.1.1.4. Foraminotomy
- 5.1.1.5. Laminotomy
- 5.1.2. Spinal Fusion
- 5.1.2.1. Cervical Fusion
- 5.1.2.2. Interbody Fusion
- 5.1.2.3. Thoraco Lumbar Fusion
- 5.1.2.4. Other Spinal Fusions
- 5.1.3. Fracture Repair
- 5.1.4. Arthroplasty
- 5.1.5. Non-fusion Procedures
- 5.1.1. Spinal Decompression
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. Germany
- 5.2.2. United Kingdom
- 5.2.3. France
- 5.2.4. Italy
- 5.2.5. Spain
- 5.2.6. Rest of Europe
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 6. Germany Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Device Type
- 6.1.1. Spinal Decompression
- 6.1.1.1. Corpectomy
- 6.1.1.2. Discectomy
- 6.1.1.3. Facetectomy
- 6.1.1.4. Foraminotomy
- 6.1.1.5. Laminotomy
- 6.1.2. Spinal Fusion
- 6.1.2.1. Cervical Fusion
- 6.1.2.2. Interbody Fusion
- 6.1.2.3. Thoraco Lumbar Fusion
- 6.1.2.4. Other Spinal Fusions
- 6.1.3. Fracture Repair
- 6.1.4. Arthroplasty
- 6.1.5. Non-fusion Procedures
- 6.1.1. Spinal Decompression
- 6.1. Market Analysis, Insights and Forecast - by Device Type
- 7. United Kingdom Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Device Type
- 7.1.1. Spinal Decompression
- 7.1.1.1. Corpectomy
- 7.1.1.2. Discectomy
- 7.1.1.3. Facetectomy
- 7.1.1.4. Foraminotomy
- 7.1.1.5. Laminotomy
- 7.1.2. Spinal Fusion
- 7.1.2.1. Cervical Fusion
- 7.1.2.2. Interbody Fusion
- 7.1.2.3. Thoraco Lumbar Fusion
- 7.1.2.4. Other Spinal Fusions
- 7.1.3. Fracture Repair
- 7.1.4. Arthroplasty
- 7.1.5. Non-fusion Procedures
- 7.1.1. Spinal Decompression
- 7.1. Market Analysis, Insights and Forecast - by Device Type
- 8. France Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Device Type
- 8.1.1. Spinal Decompression
- 8.1.1.1. Corpectomy
- 8.1.1.2. Discectomy
- 8.1.1.3. Facetectomy
- 8.1.1.4. Foraminotomy
- 8.1.1.5. Laminotomy
- 8.1.2. Spinal Fusion
- 8.1.2.1. Cervical Fusion
- 8.1.2.2. Interbody Fusion
- 8.1.2.3. Thoraco Lumbar Fusion
- 8.1.2.4. Other Spinal Fusions
- 8.1.3. Fracture Repair
- 8.1.4. Arthroplasty
- 8.1.5. Non-fusion Procedures
- 8.1.1. Spinal Decompression
- 8.1. Market Analysis, Insights and Forecast - by Device Type
- 9. Italy Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Device Type
- 9.1.1. Spinal Decompression
- 9.1.1.1. Corpectomy
- 9.1.1.2. Discectomy
- 9.1.1.3. Facetectomy
- 9.1.1.4. Foraminotomy
- 9.1.1.5. Laminotomy
- 9.1.2. Spinal Fusion
- 9.1.2.1. Cervical Fusion
- 9.1.2.2. Interbody Fusion
- 9.1.2.3. Thoraco Lumbar Fusion
- 9.1.2.4. Other Spinal Fusions
- 9.1.3. Fracture Repair
- 9.1.4. Arthroplasty
- 9.1.5. Non-fusion Procedures
- 9.1.1. Spinal Decompression
- 9.1. Market Analysis, Insights and Forecast - by Device Type
- 10. Spain Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Device Type
- 10.1.1. Spinal Decompression
- 10.1.1.1. Corpectomy
- 10.1.1.2. Discectomy
- 10.1.1.3. Facetectomy
- 10.1.1.4. Foraminotomy
- 10.1.1.5. Laminotomy
- 10.1.2. Spinal Fusion
- 10.1.2.1. Cervical Fusion
- 10.1.2.2. Interbody Fusion
- 10.1.2.3. Thoraco Lumbar Fusion
- 10.1.2.4. Other Spinal Fusions
- 10.1.3. Fracture Repair
- 10.1.4. Arthroplasty
- 10.1.5. Non-fusion Procedures
- 10.1.1. Spinal Decompression
- 10.1. Market Analysis, Insights and Forecast - by Device Type
- 11. Rest of Europe Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 11.1. Market Analysis, Insights and Forecast - by Device Type
- 11.1.1. Spinal Decompression
- 11.1.1.1. Corpectomy
- 11.1.1.2. Discectomy
- 11.1.1.3. Facetectomy
- 11.1.1.4. Foraminotomy
- 11.1.1.5. Laminotomy
- 11.1.2. Spinal Fusion
- 11.1.2.1. Cervical Fusion
- 11.1.2.2. Interbody Fusion
- 11.1.2.3. Thoraco Lumbar Fusion
- 11.1.2.4. Other Spinal Fusions
- 11.1.3. Fracture Repair
- 11.1.4. Arthroplasty
- 11.1.5. Non-fusion Procedures
- 11.1.1. Spinal Decompression
- 11.1. Market Analysis, Insights and Forecast - by Device Type
- 12. Germany Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 13. United Kingdom Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 14. France Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 15. Italy Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 16. Spain Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 17. Rest of Europe Europe Spinal Surgery Devices Industry Analysis, Insights and Forecast, 2019-2031
- 18. Competitive Analysis
- 18.1. Market Share Analysis 2024
- 18.2. Company Profiles
- 18.2.1 ZimVie Inc
- 18.2.1.1. Overview
- 18.2.1.2. Products
- 18.2.1.3. SWOT Analysis
- 18.2.1.4. Recent Developments
- 18.2.1.5. Financials (Based on Availability)
- 18.2.2 Orthofix Holdings Inc
- 18.2.2.1. Overview
- 18.2.2.2. Products
- 18.2.2.3. SWOT Analysis
- 18.2.2.4. Recent Developments
- 18.2.2.5. Financials (Based on Availability)
- 18.2.3 Alphatec Spine Inc
- 18.2.3.1. Overview
- 18.2.3.2. Products
- 18.2.3.3. SWOT Analysis
- 18.2.3.4. Recent Developments
- 18.2.3.5. Financials (Based on Availability)
- 18.2.4 Depuy Synthes Spine Inc (Johnson & Johnson)
- 18.2.4.1. Overview
- 18.2.4.2. Products
- 18.2.4.3. SWOT Analysis
- 18.2.4.4. Recent Developments
- 18.2.4.5. Financials (Based on Availability)
- 18.2.5 Globus Medical Inc
- 18.2.5.1. Overview
- 18.2.5.2. Products
- 18.2.5.3. SWOT Analysis
- 18.2.5.4. Recent Developments
- 18.2.5.5. Financials (Based on Availability)
- 18.2.6 Medtronic PLC
- 18.2.6.1. Overview
- 18.2.6.2. Products
- 18.2.6.3. SWOT Analysis
- 18.2.6.4. Recent Developments
- 18.2.6.5. Financials (Based on Availability)
- 18.2.7 SeaSpine Inc
- 18.2.7.1. Overview
- 18.2.7.2. Products
- 18.2.7.3. SWOT Analysis
- 18.2.7.4. Recent Developments
- 18.2.7.5. Financials (Based on Availability)
- 18.2.8 SpineGuard SA
- 18.2.8.1. Overview
- 18.2.8.2. Products
- 18.2.8.3. SWOT Analysis
- 18.2.8.4. Recent Developments
- 18.2.8.5. Financials (Based on Availability)
- 18.2.9 Stryker Corporation
- 18.2.9.1. Overview
- 18.2.9.2. Products
- 18.2.9.3. SWOT Analysis
- 18.2.9.4. Recent Developments
- 18.2.9.5. Financials (Based on Availability)
- 18.2.10 NuVasive Inc
- 18.2.10.1. Overview
- 18.2.10.2. Products
- 18.2.10.3. SWOT Analysis
- 18.2.10.4. Recent Developments
- 18.2.10.5. Financials (Based on Availability)
- 18.2.1 ZimVie Inc
List of Figures
- Figure 1: Europe Spinal Surgery Devices Industry Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Europe Spinal Surgery Devices Industry Share (%) by Company 2024
List of Tables
- Table 1: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Region 2019 & 2032
- Table 3: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 4: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 5: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 6: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Region 2019 & 2032
- Table 7: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 8: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 9: Germany Europe Spinal Surgery Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 10: Germany Europe Spinal Surgery Devices Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 11: United Kingdom Europe Spinal Surgery Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 12: United Kingdom Europe Spinal Surgery Devices Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 13: France Europe Spinal Surgery Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 14: France Europe Spinal Surgery Devices Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 15: Italy Europe Spinal Surgery Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 16: Italy Europe Spinal Surgery Devices Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 17: Spain Europe Spinal Surgery Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 18: Spain Europe Spinal Surgery Devices Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 19: Rest of Europe Europe Spinal Surgery Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 20: Rest of Europe Europe Spinal Surgery Devices Industry Volume (K Units) Forecast, by Application 2019 & 2032
- Table 21: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 22: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 23: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 24: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 25: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 26: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 27: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 28: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 29: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 30: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 31: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 32: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 33: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 34: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 35: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 36: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 37: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 38: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 39: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 40: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Country 2019 & 2032
- Table 41: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Device Type 2019 & 2032
- Table 42: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Device Type 2019 & 2032
- Table 43: Europe Spinal Surgery Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 44: Europe Spinal Surgery Devices Industry Volume K Units Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Europe Spinal Surgery Devices Industry?
The projected CAGR is approximately 6.30%.
2. Which companies are prominent players in the Europe Spinal Surgery Devices Industry?
Key companies in the market include ZimVie Inc , Orthofix Holdings Inc, Alphatec Spine Inc, Depuy Synthes Spine Inc (Johnson & Johnson), Globus Medical Inc, Medtronic PLC, SeaSpine Inc, SpineGuard SA, Stryker Corporation, NuVasive Inc.
3. What are the main segments of the Europe Spinal Surgery Devices Industry?
The market segments include Device Type.
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Demand for Minimally Invasive Surgical Procedures; Increasing Incidence of Obesity. Aging Population. and Associated Spine Disorders; Continuous Advancements in Spine Surgery Technologies.
6. What are the notable trends driving market growth?
Lumbar Fusion is Expected to Witness a Significant Growth Over the Forecast Period.
7. Are there any restraints impacting market growth?
High Cost and Time Involved in Treatment Procedures; Reimbursement Issues.
8. Can you provide examples of recent developments in the market?
July 2022: Spineway, a specialist in innovative implants for the treatment of severe spine pathologies, completed the acquisition of 100% of the capital of the French company Spine Innovations, which specializes in cervical and lumbar disc prostheses.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Units.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Europe Spinal Surgery Devices Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Europe Spinal Surgery Devices Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Europe Spinal Surgery Devices Industry?
To stay informed about further developments, trends, and reports in the Europe Spinal Surgery Devices Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


