Key Insights
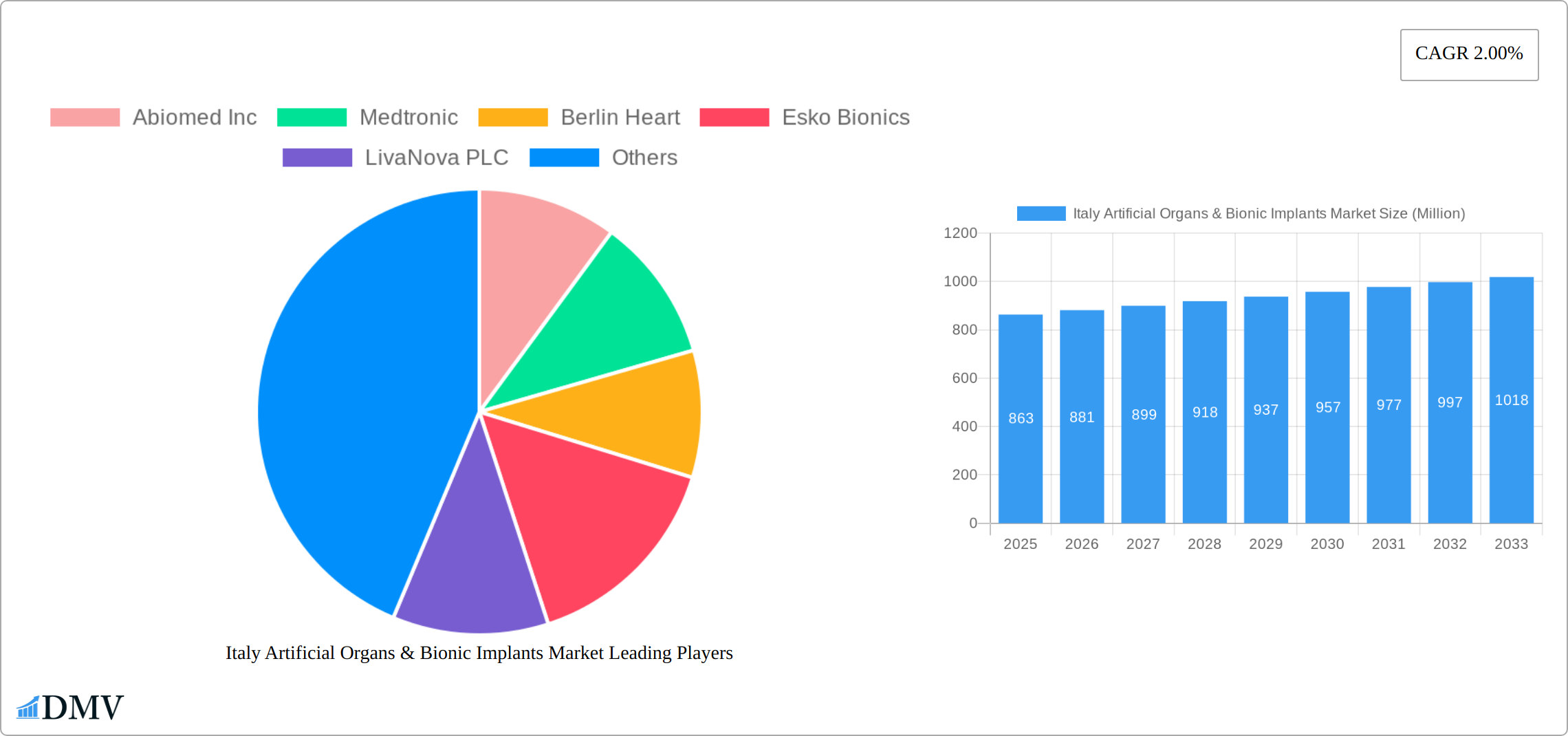
The Italy Artificial Organs & Bionic Implants Market, valued at €863 million in 2025, is projected to experience steady growth, driven by a rising geriatric population, increasing prevalence of chronic diseases requiring organ replacement or augmentation, and advancements in medical technology leading to more sophisticated and effective implants. The market's Compound Annual Growth Rate (CAGR) of 2.00% from 2025 to 2033 indicates a consistent, albeit moderate, expansion. Key market segments include artificial organs (such as heart pumps, kidney dialysis machines, and artificial lungs) and bionic implants (including cochlear implants, prosthetic limbs, and retinal implants). The market is largely influenced by technological innovation, regulatory approvals, and reimbursement policies. Competition is fierce, with major players like Abiomed Inc, Medtronic, and others actively investing in R&D and strategic acquisitions to expand their market share within Italy. The success of these companies is dependent on their ability to develop cutting-edge technology, secure regulatory approvals, and establish strong distribution networks to reach healthcare providers and patients across the country. Furthermore, the increasing focus on personalized medicine and minimally invasive surgical techniques is expected to further fuel market growth in the coming years.
The segment of artificial organs is likely to hold a larger market share due to the high prevalence of conditions requiring organ replacement. However, the bionic implants segment is anticipated to witness faster growth owing to continuous technological improvements and increased affordability. Government initiatives promoting advancements in healthcare technology, alongside rising healthcare expenditure, contribute to a favorable market environment. However, potential restraints include the high cost of these devices, stringent regulatory requirements, and concerns regarding long-term efficacy and potential complications. The market's trajectory will be shaped by the evolving regulatory landscape, technological advancements, and shifts in healthcare spending patterns within Italy.
Italy Artificial Organs & Bionic Implants Market Market Composition & Trends
The Italy Artificial Organs & Bionic Implants Market is characterized by a dynamic interplay of innovation, regulatory frameworks, and market consolidation. Market concentration remains moderate, with a few key players like Medtronic and Abiomed Inc holding significant shares, estimated at 25% and 15% respectively. The catalyst for innovation in this sector includes advancements in materials science and robotics, driving the development of more efficient and durable artificial organs and bionic implants.
Regulatory landscapes in Italy are stringent, with the European Union Medical Device Regulation (MDR) setting high standards for safety and efficacy. This has led to a cautious approach in product launches, with companies investing heavily in compliance. Substitute products, such as pharmaceutical treatments, pose a competitive threat, though the unique benefits of artificial organs and bionic implants maintain their market edge.
End-user profiles primarily consist of hospitals and specialized clinics, with a growing interest from home healthcare settings. The demand for personalized healthcare solutions is increasing, influencing the development of tailored bionic implants.
Mergers and acquisitions are pivotal in shaping the market landscape. For instance, a notable M&A deal in 2022 valued at EUR 50 Million saw a major player acquiring a smaller competitor to expand its product portfolio and market reach.
Market Share Distribution:
Medtronic: 25%
Abiomed Inc: 15%
Others: 60%
M&A Deal Values:
2022 Acquisition: EUR 50 Million
Italy Artificial Organs & Bionic Implants Market Industry Evolution
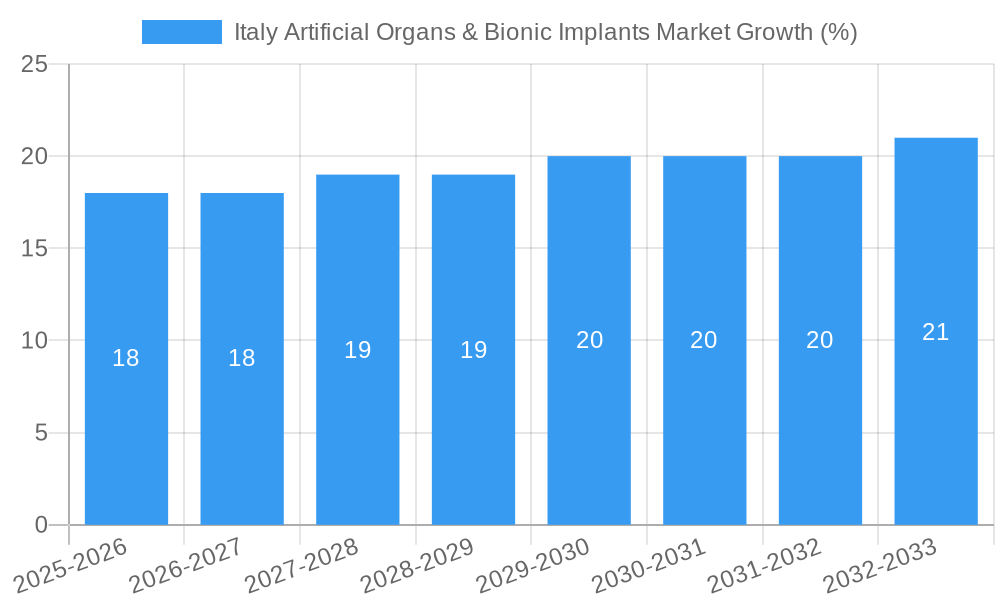
The Italian artificial organs and bionic implants market has demonstrated robust growth, achieving a compound annual growth rate (CAGR) of 7.2% between 2019 and 2024. This expansion is primarily fueled by remarkable technological advancements, leading to the development of increasingly sophisticated artificial organs and bionic implants. The seamless integration of AI and IoT technologies allows for real-time monitoring and adjustments of these devices, resulting in significantly improved patient outcomes and a higher quality of life.
The adoption of bionic implants has seen a substantial surge, with a notable 10% annual increase in implantation rates over the past five years. This upward trend is largely attributed to Italy's aging population and the rising prevalence of chronic diseases, creating a greater demand for advanced medical solutions. Furthermore, evolving consumer preferences are driving the market towards minimally invasive procedures and devices that promote greater mobility and independence for patients.
Within the artificial organ segment, heart and lung devices have witnessed the most significant progress, with newer models exhibiting improved longevity and markedly reduced rejection rates. This market evolution is also significantly influenced by increased investment in research and development (R&D), with leading companies such as LivaNova PLC and Berlin Heart spearheading the innovation in pioneering new technologies and treatment options.
The regulatory landscape in Italy is also dynamically evolving. Recent updates to the Medical Device Regulation (MDR) have streamlined the market entry process for innovative products while upholding stringent safety standards. This balanced approach is crucial for ensuring the sustained growth and widespread acceptance of artificial organs and bionic implants within the Italian healthcare system.
Leading Regions, Countries, or Segments in Italy Artificial Organs & Bionic Implants Market
The artificial organ segment currently dominates the Italian market, driven by the critical need for heart and lung replacements. This segment's growth is fueled by several key factors:
Robust Investment Trends: Significant R&D investments are being made by leading multinational corporations such as Medtronic and Abiomed Inc., alongside substantial government funding allocated to healthcare innovation, particularly within the Lombardy region.
Supportive Regulatory Environment: Favorable policies under the European Union Medical Device Regulation (MDR) expedite the approval of new medical devices. This includes streamlined processes for clinical trials and patient enrollment, accelerating the time to market for innovative solutions.
The prominence of the artificial organ segment stems from several interconnected factors. The high prevalence of cardiovascular diseases in Italy necessitates advanced solutions like artificial hearts. The Italian healthcare system's commitment to high-quality care and cutting-edge technology creates a supportive environment for the adoption of these life-saving devices. Furthermore, collaborative partnerships between leading academic institutions and industry players have significantly accelerated the development and successful commercialization of artificial organs.
While the bionic implants segment is experiencing growth, it faces challenges related to higher costs and the need for specialized surgical expertise. However, advancements in bionic limbs and cochlear implants are creating new avenues for market expansion, particularly in regions like Tuscany, known for its strong focus on rehabilitation and assistive technologies. Government initiatives and public awareness campaigns promoting assistive technologies are also expected to contribute to market growth in this segment.
Italy Artificial Organs & Bionic Implants Market Product Innovations
Groundbreaking innovations are transforming patient care within the Italian artificial organs and bionic implants market. Recent advancements include the utilization of 3D printing technology for the creation of bioengineered organs, offering customized solutions with significantly reduced rejection rates. Bionic implants, such as advanced prosthetic limbs, now incorporate sophisticated neural interfaces that enhance user control, feedback, and overall functionality. These innovations not only improve device performance but also dramatically enhance the quality of life for patients, establishing new benchmarks within the medical device industry.
Propelling Factors for Italy Artificial Organs & Bionic Implants Market Growth
Several factors are driving the growth of the Italy Artificial Organs & Bionic Implants Market. Technologically, advancements in biomaterials and robotics are enabling the creation of more effective devices. Economically, Italy's healthcare spending, which reached EUR 150 Billion in 2022, supports the adoption of high-cost medical technologies. Regulatory influences, such as the streamlined approval processes under the MDR, facilitate quicker market entry for innovative products, further propelling market growth.
Obstacles in the Italy Artificial Organs & Bionic Implants Market Market
The Italy Artificial Organs & Bionic Implants Market faces several obstacles. Regulatory challenges, particularly the stringent requirements of the MDR, can delay product launches and increase costs. Supply chain disruptions, exacerbated by global events, have led to a 15% increase in production delays. Competitive pressures are intense, with new entrants offering lower-cost alternatives, impacting market share and pricing strategies.
Future Opportunities in Italy Artificial Organs & Bionic Implants Market
Emerging opportunities in the Italy Artificial Organs & Bionic Implants Market include the expansion into new markets like telemedicine and home healthcare. Technological advancements, such as the integration of AI for predictive maintenance of implants, are poised to revolutionize patient care. Consumer trends towards personalized medicine are also opening new avenues for growth, particularly in the customization of bionic implants.
Major Players in the Italy Artificial Organs & Bionic Implants Market Ecosystem
Key Developments in Italy Artificial Organs & Bionic Implants Market Industry
February 2023: Pixium Vision SA secured a EUR 7.7 Million cash position, extending its operational runway through Q2 2023. This funding bolstered the company's pivotal PRIMAvera trial for atrophic dry age-related macular degeneration (dry AMD) in Italy, highlighting continued investment in vision restoration technologies within the bionic implants sector.
September 2022: The establishment of a landmark partnership between the Alliance for Paired Kidney Donation (APKD) and Centro Nazionali Trapianti (CNT) enabled the first-ever living donor kidney exchange between the United States and Italy. This collaboration has significant implications for the artificial organ market, fostering international cooperation and increasing the availability of organs for transplantation.
Strategic Italy Artificial Organs & Bionic Implants Market Market Forecast
The Italy Artificial Organs & Bionic Implants Market is poised for robust growth over the forecast period of 2025-2033, driven by technological innovations, increasing healthcare expenditure, and favorable regulatory environments. The integration of AI and IoT in medical devices will continue to enhance patient outcomes and device efficiency. Additionally, the expansion into telemedicine and home healthcare presents significant opportunities for market expansion. With a projected CAGR of 8.5%, the market is set to reach new heights, offering substantial potential for stakeholders.
Study Period: 2019–2033 Base Year: 2025 Estimated Year: 2025 Forecast Period: 2025–2033 Historical Period: 2019–2024
Italy Artificial Organs & Bionic Implants Market Segmentation
-
1. Type
-
1.1. Artificial Organ
- 1.1.1. Artificial Heart
- 1.1.2. Artificial Kidney
- 1.1.3. Cochlear Implants
- 1.1.4. Other Organ Types
-
1.2. Bionics
- 1.2.1. Ear Bionics
- 1.2.2. Orthopedic Bionic
- 1.2.3. Cardiac Bionics
- 1.2.4. Eye Bionics
- 1.2.5. Others
-
1.1. Artificial Organ
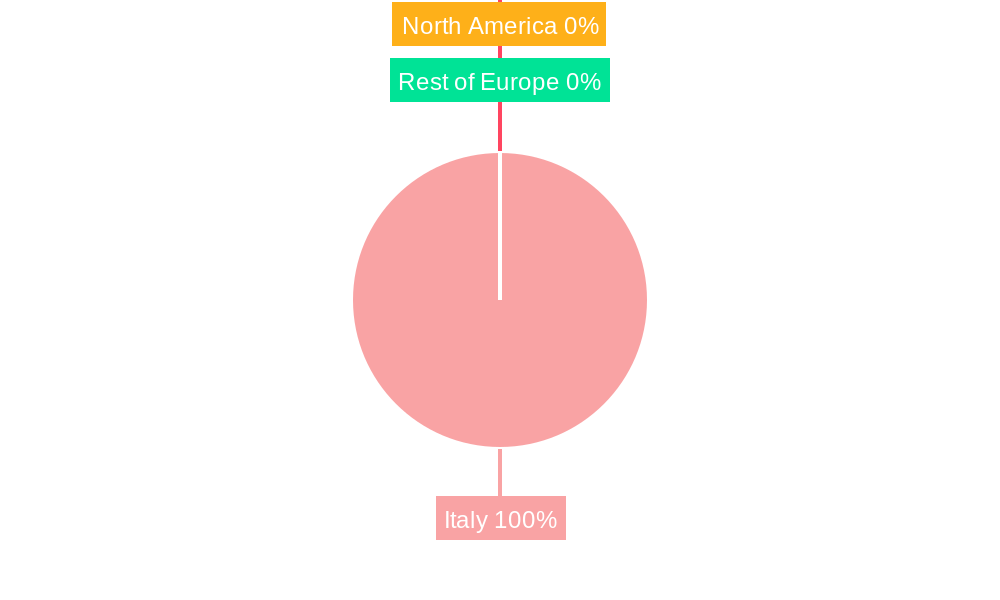
Italy Artificial Organs & Bionic Implants Market Segmentation By Geography
- 1. Italy
Italy Artificial Organs & Bionic Implants Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 2.00% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1 Increased Incidence of Disabilities
- 3.2.2 Organ Failures and Scarcity of Donors; High Incidence of Road Accidents Leading to Amputations; Technological Advancements in the Artificial Organ and Bionics
- 3.3. Market Restrains
- 3.3.1. Expensive Procedures; Risk of Compatibility Issues and Malfunctions
- 3.4. Market Trends
- 3.4.1. Eye Bionics Segment is Estimated to Witness a Healthy Growth Over the Forecast Period.
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Italy Artificial Organs & Bionic Implants Market Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Type
- 5.1.1. Artificial Organ
- 5.1.1.1. Artificial Heart
- 5.1.1.2. Artificial Kidney
- 5.1.1.3. Cochlear Implants
- 5.1.1.4. Other Organ Types
- 5.1.2. Bionics
- 5.1.2.1. Ear Bionics
- 5.1.2.2. Orthopedic Bionic
- 5.1.2.3. Cardiac Bionics
- 5.1.2.4. Eye Bionics
- 5.1.2.5. Others
- 5.1.1. Artificial Organ
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. Italy
- 5.1. Market Analysis, Insights and Forecast - by Type
- 6. Competitive Analysis
- 6.1. Market Share Analysis 2024
- 6.2. Company Profiles
- 6.2.1 Abiomed Inc
- 6.2.1.1. Overview
- 6.2.1.2. Products
- 6.2.1.3. SWOT Analysis
- 6.2.1.4. Recent Developments
- 6.2.1.5. Financials (Based on Availability)
- 6.2.2 Medtronic
- 6.2.2.1. Overview
- 6.2.2.2. Products
- 6.2.2.3. SWOT Analysis
- 6.2.2.4. Recent Developments
- 6.2.2.5. Financials (Based on Availability)
- 6.2.3 Berlin Heart
- 6.2.3.1. Overview
- 6.2.3.2. Products
- 6.2.3.3. SWOT Analysis
- 6.2.3.4. Recent Developments
- 6.2.3.5. Financials (Based on Availability)
- 6.2.4 Esko Bionics
- 6.2.4.1. Overview
- 6.2.4.2. Products
- 6.2.4.3. SWOT Analysis
- 6.2.4.4. Recent Developments
- 6.2.4.5. Financials (Based on Availability)
- 6.2.5 LivaNova PLC
- 6.2.5.1. Overview
- 6.2.5.2. Products
- 6.2.5.3. SWOT Analysis
- 6.2.5.4. Recent Developments
- 6.2.5.5. Financials (Based on Availability)
- 6.2.6 Cochlear Ltd
- 6.2.6.1. Overview
- 6.2.6.2. Products
- 6.2.6.3. SWOT Analysis
- 6.2.6.4. Recent Developments
- 6.2.6.5. Financials (Based on Availability)
- 6.2.7 Össur
- 6.2.7.1. Overview
- 6.2.7.2. Products
- 6.2.7.3. SWOT Analysis
- 6.2.7.4. Recent Developments
- 6.2.7.5. Financials (Based on Availability)
- 6.2.8 Getinge AB
- 6.2.8.1. Overview
- 6.2.8.2. Products
- 6.2.8.3. SWOT Analysis
- 6.2.8.4. Recent Developments
- 6.2.8.5. Financials (Based on Availability)
- 6.2.1 Abiomed Inc
List of Figures
- Figure 1: Italy Artificial Organs & Bionic Implants Market Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Italy Artificial Organs & Bionic Implants Market Share (%) by Company 2024
List of Tables
- Table 1: Italy Artificial Organs & Bionic Implants Market Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Italy Artificial Organs & Bionic Implants Market Volume Piece Forecast, by Region 2019 & 2032
- Table 3: Italy Artificial Organs & Bionic Implants Market Revenue Million Forecast, by Type 2019 & 2032
- Table 4: Italy Artificial Organs & Bionic Implants Market Volume Piece Forecast, by Type 2019 & 2032
- Table 5: Italy Artificial Organs & Bionic Implants Market Revenue Million Forecast, by Region 2019 & 2032
- Table 6: Italy Artificial Organs & Bionic Implants Market Volume Piece Forecast, by Region 2019 & 2032
- Table 7: Italy Artificial Organs & Bionic Implants Market Revenue Million Forecast, by Country 2019 & 2032
- Table 8: Italy Artificial Organs & Bionic Implants Market Volume Piece Forecast, by Country 2019 & 2032
- Table 9: Italy Artificial Organs & Bionic Implants Market Revenue Million Forecast, by Type 2019 & 2032
- Table 10: Italy Artificial Organs & Bionic Implants Market Volume Piece Forecast, by Type 2019 & 2032
- Table 11: Italy Artificial Organs & Bionic Implants Market Revenue Million Forecast, by Country 2019 & 2032
- Table 12: Italy Artificial Organs & Bionic Implants Market Volume Piece Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Italy Artificial Organs & Bionic Implants Market?
The projected CAGR is approximately 2.00%.
2. Which companies are prominent players in the Italy Artificial Organs & Bionic Implants Market?
Key companies in the market include Abiomed Inc, Medtronic, Berlin Heart, Esko Bionics, LivaNova PLC, Cochlear Ltd, Össur, Getinge AB.
3. What are the main segments of the Italy Artificial Organs & Bionic Implants Market?
The market segments include Type.
4. Can you provide details about the market size?
The market size is estimated to be USD 863 Million as of 2022.
5. What are some drivers contributing to market growth?
Increased Incidence of Disabilities. Organ Failures and Scarcity of Donors; High Incidence of Road Accidents Leading to Amputations; Technological Advancements in the Artificial Organ and Bionics.
6. What are the notable trends driving market growth?
Eye Bionics Segment is Estimated to Witness a Healthy Growth Over the Forecast Period..
7. Are there any restraints impacting market growth?
Expensive Procedures; Risk of Compatibility Issues and Malfunctions.
8. Can you provide examples of recent developments in the market?
In February 2023, Pixium Vision SA received a cash position of EUR 7.7 million in December 2022, providing an estimated cash runway through the end of Q2 2023. The increased cash outflow was primarily due to the enrolment and implantation of patients with the PRIMAvera pivotal trial for atrophic dry age-related macular degeneration (dry AMD) in Italy.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in Piece.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Italy Artificial Organs & Bionic Implants Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Italy Artificial Organs & Bionic Implants Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Italy Artificial Organs & Bionic Implants Market?
To stay informed about further developments, trends, and reports in the Italy Artificial Organs & Bionic Implants Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


