Key Insights
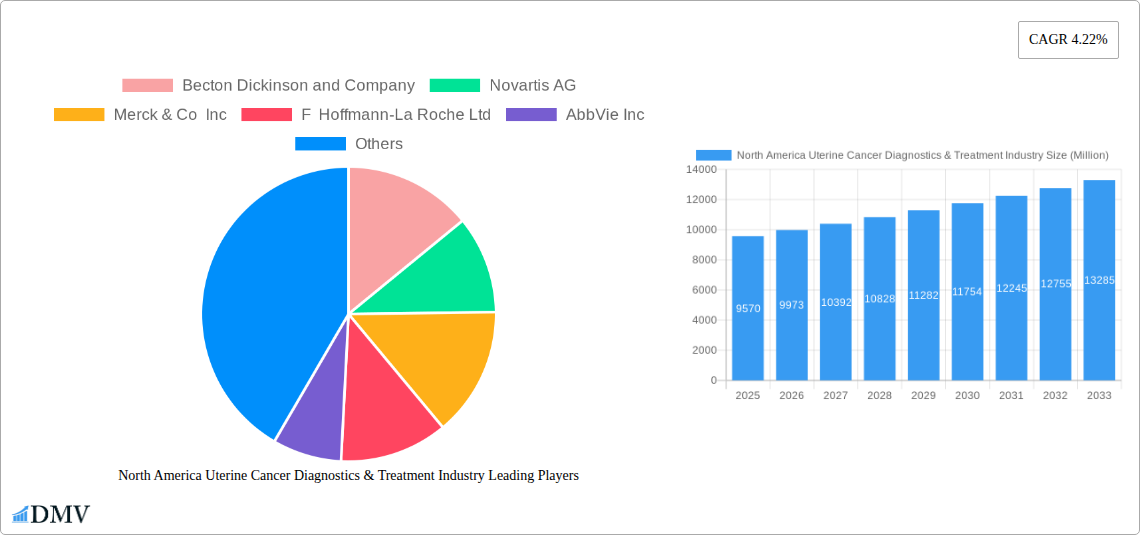
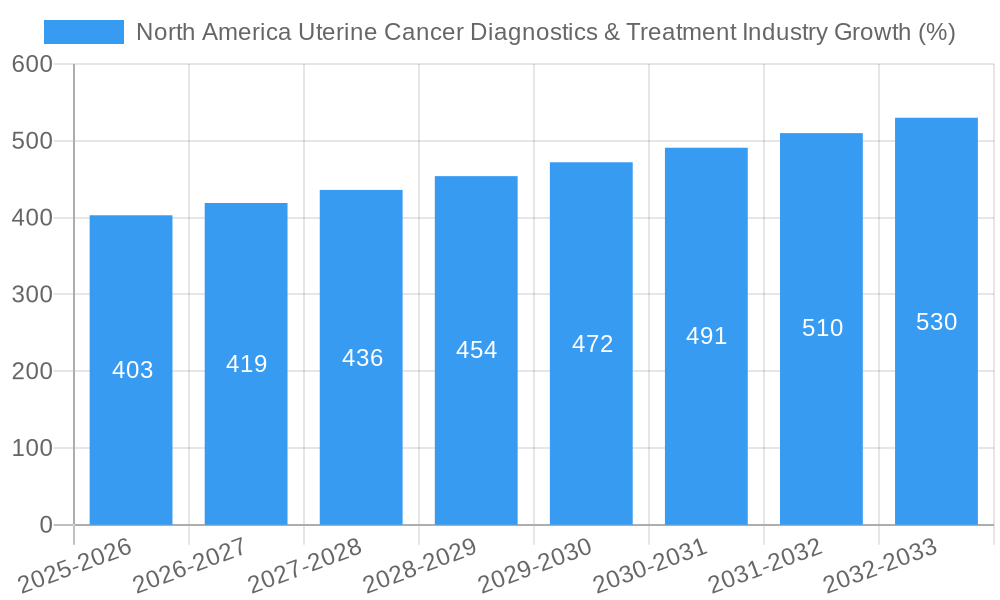
The North American uterine cancer diagnostics and treatment market, valued at $9.57 billion in 2025, is projected to experience robust growth, driven by rising incidence rates of endometrial cancer and uterine sarcoma, advancements in diagnostic technologies (like advanced imaging and molecular diagnostics), and the development of targeted therapies. The market's Compound Annual Growth Rate (CAGR) of 4.22% from 2019-2033 indicates a steady expansion, with significant contributions expected from both treatment and diagnostic segments. Growth is fueled by an aging population increasing susceptibility to these cancers, increased healthcare spending, and rising awareness leading to earlier diagnosis and treatment. While the specific breakdown of market share by cancer type (endometrial vs. uterine sarcoma) and procedure (treatment vs. diagnostics) isn't provided, it's reasonable to assume a significant portion is dedicated to treatment given the higher cost and complexity of therapies compared to diagnostics. The dominance of major pharmaceutical companies like Merck, Roche, and Novartis reflects the substantial investment in research and development within this sector. The continued development of novel therapies, including immunotherapy and targeted agents, promises to further propel market expansion.
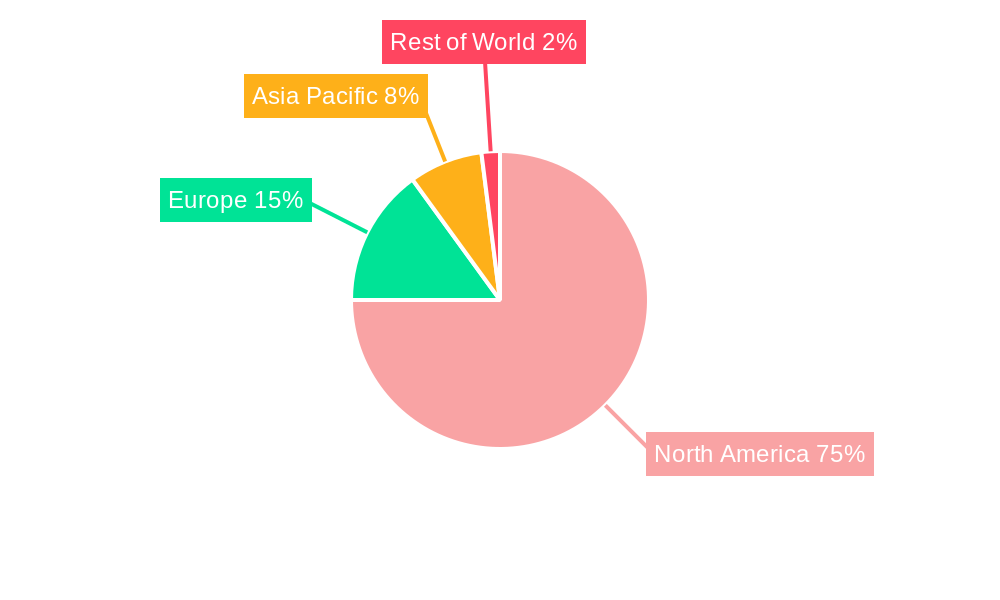
The market's geographic concentration within North America, particularly the United States, is expected to remain strong due to advanced healthcare infrastructure, higher disposable incomes, and substantial research funding. However, the Canadian and Mexican markets will also contribute to overall growth, albeit at potentially slower rates. Regulatory approvals for new drugs and diagnostics will remain a crucial factor influencing market dynamics. While potential restraints like high treatment costs and access issues may exist, the overall positive trajectory suggests considerable opportunities for industry players throughout the forecast period (2025-2033). The increasing prevalence of minimally invasive surgical techniques and a greater focus on personalized medicine are also likely to shape the future of the market.
North America Uterine Cancer Diagnostics & Treatment Industry: A Comprehensive Market Report (2019-2033)
This insightful report provides a detailed analysis of the North America uterine cancer diagnostics and treatment industry, offering a comprehensive overview of market dynamics, key players, and future growth prospects. Covering the period from 2019 to 2033, with a base year of 2025 and a forecast period of 2025-2033, this report is an essential resource for stakeholders seeking to understand and capitalize on opportunities within this evolving sector. The market size in 2025 is estimated at xx Million, projected to reach xx Million by 2033.
North America Uterine Cancer Diagnostics & Treatment Industry Market Composition & Trends
This section delves into the competitive landscape of the North America uterine cancer diagnostics and treatment market, evaluating market concentration, innovation, regulatory factors, and market activity. The report examines the market share distribution among key players, analyzing their strategies and competitive advantages. Significant mergers and acquisitions (M&A) within the industry are also scrutinized, including deal values and their impact on market consolidation. The report further explores the influence of substitute products, end-user profiles (hospitals, clinics, etc.), and the ever-evolving regulatory landscape. The analysis considers the impact of these factors on market growth and future trends, providing valuable insights for strategic decision-making.
- Market Concentration: The market exhibits a [High/Medium/Low] level of concentration, with [Percentage]% controlled by the top [Number] players.
- Innovation Catalysts: Ongoing research into novel therapies, advanced diagnostic tools, and personalized medicine are driving innovation.
- Regulatory Landscape: FDA approvals and guidelines significantly influence market access and product development.
- Substitute Products: [Mention any existing or potential substitute products and their impact].
- End-User Profiles: The market is primarily driven by hospitals and specialized oncology clinics.
- M&A Activities: Significant M&A activity has been observed, with [Number] deals valued at approximately xx Million in the last [Number] years. Examples include [mention specific examples if available].
North America Uterine Cancer Diagnostics & Treatment Industry Industry Evolution
This section provides a detailed analysis of the historical and projected growth trajectories of the North American uterine cancer diagnostics and treatment market. It examines the factors influencing market expansion, including technological advancements in diagnostics and therapeutics, evolving treatment paradigms, and shifts in patient demographics and healthcare spending. Specific data points, including compound annual growth rates (CAGR) for both the historical (2019-2024) and forecast (2025-2033) periods, are included. The analysis also assesses the adoption rates of new technologies and treatment approaches, highlighting the key trends shaping the industry's evolution. The report includes a detailed examination of the impact of technological advancements like immunotherapy and targeted therapies on market growth.
Leading Regions, Countries, or Segments in North America Uterine Cancer Diagnostics & Treatment Industry
This section identifies the leading regions, countries, and segments within the North American uterine cancer diagnostics and treatment market. It analyzes the factors contributing to the dominance of these specific areas. The analysis considers both cancer types (Endometrial Cancer, Uterine Sarcoma) and procedures (Treatment, Diagnostics).
- By Cancer Type:
- Endometrial Cancer: [Dominant region/country]. Key drivers include [List bullet points: e.g., higher incidence rates, increased awareness, targeted therapies].
- Uterine Sarcoma: [Dominant region/country]. Key drivers include [List bullet points: e.g., advancements in targeted therapies, improved diagnostic techniques].
- By Procedure:
- Treatment: [Dominant region/country]. Factors include [Paragraph explaining dominance factors, e.g., higher healthcare expenditure, better access to advanced treatments].
- Diagnostics: [Dominant region/country]. Factors include [Paragraph explaining dominance factors, e.g., increased screening rates, early diagnosis initiatives].
North America Uterine Cancer Diagnostics & Treatment Industry Product Innovations
The North American uterine cancer diagnostics and treatment industry is characterized by ongoing innovation in both diagnostic tools and therapeutic approaches. Recent advancements include the development of more sensitive and specific diagnostic tests, personalized medicine approaches that tailor treatment to individual patient characteristics, and innovative therapies such as immunotherapies and targeted agents that offer improved efficacy and reduced side effects. These innovations are improving patient outcomes and transforming the landscape of uterine cancer management.
Propelling Factors for North America Uterine Cancer Diagnostics & Treatment Industry Growth
Several factors contribute to the growth of the North American uterine cancer diagnostics and treatment market. These include: increasing prevalence of uterine cancers, advancements in diagnostic technologies leading to earlier detection, development of novel and more effective therapies, rising healthcare expenditure, and increasing government initiatives to improve cancer care. The increasing adoption of advanced imaging techniques and molecular diagnostics further fuels market expansion.
Obstacles in the North America Uterine Cancer Diagnostics & Treatment Industry Market
Despite significant progress, several challenges hinder market growth. High cost of innovative therapies can limit accessibility, particularly for underserved populations. Furthermore, reimbursement policies and regulatory hurdles can delay market entry of new products. Supply chain disruptions and the complexities of clinical trials add to the existing challenges. Competition amongst established players also intensifies market dynamics.
Future Opportunities in North America Uterine Cancer Diagnostics & Treatment Industry
Future opportunities lie in the development and adoption of personalized medicine, liquid biopsies for early detection, novel targeted therapies, and improved supportive care options. Expanding access to high-quality care in underserved areas presents a significant opportunity. Focus on innovative diagnostic tools and minimally invasive treatment options will further drive market expansion.
Major Players in the North America Uterine Cancer Diagnostics & Treatment Industry Ecosystem
- Becton Dickinson and Company
- Novartis AG
- Merck & Co Inc
- F Hoffmann-La Roche Ltd
- AbbVie Inc
- Takeda Pharmaceutical Company Limited
- Bristol-Myers Squibb Company
- GlaxoSmithKline PLC
- Pfizer Inc
Key Developments in North America Uterine Cancer Diagnostics & Treatment Industry Industry
- February 2023: GSK announced FDA full approval for Jemperli (dostarlimab-gxly) for recurrent or advanced mismatch repair-deficient (dMMR) endometrial cancer. This significantly expands treatment options for this patient population.
- March 2022: FDA approval of pembrolizumab (Keytruda, Merck) as monotherapy for advanced endometrial cancer with MSI-H or dMMR after prior systemic therapy. This marks a substantial advancement in the treatment of advanced endometrial cancer.
Strategic North America Uterine Cancer Diagnostics & Treatment Industry Market Forecast
The North America uterine cancer diagnostics and treatment market is poised for substantial growth driven by technological innovation, increasing prevalence of uterine cancers, and a growing awareness of effective treatment options. The forecast period (2025-2033) will witness the introduction of several new therapies, diagnostic tools, and personalized medicine approaches. This, coupled with rising healthcare spending, will continue to propel market expansion and present lucrative opportunities for key players in the industry.
North America Uterine Cancer Diagnostics & Treatment Industry Segmentation
-
1. Cancer Type
- 1.1. Endometrial Cancer
- 1.2. Uterine Sarcoma
-
2. Procedure
-
2.1. Treatment
- 2.1.1. Surgery
- 2.1.2. Immunotherapy
- 2.1.3. Radiation Therapy
- 2.1.4. Chemotherapy
- 2.1.5. Other Treatments
-
2.2. Diagnostics
- 2.2.1. Biopsy
- 2.2.2. Ultrasound
- 2.2.3. Hysteroscopy
- 2.2.4. Dilation and Curettage
- 2.2.5. Other Diagnostics
-
2.1. Treatment
-
3. Geography
-
3.1. North America
- 3.1.1. United States
- 3.1.2. Canada
- 3.1.3. Mexico
-
3.1. North America
North America Uterine Cancer Diagnostics & Treatment Industry Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
North America Uterine Cancer Diagnostics & Treatment Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 4.22% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Rising Awareness about Uterine Diseases and the Available Therapies; Increasing Health Care Expenditure; Innovation in Drug Development and Subsequent Technological Advancements
- 3.3. Market Restrains
- 3.3.1. Low Success Rate in Clinical Trials for Cancer Drugs and High Cost of Research and Development; High Cost Associated with the Treatment
- 3.4. Market Trends
- 3.4.1. Immunotherapy Segment is Expected to Register Considerable Growth Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. North America Uterine Cancer Diagnostics & Treatment Industry Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Cancer Type
- 5.1.1. Endometrial Cancer
- 5.1.2. Uterine Sarcoma
- 5.2. Market Analysis, Insights and Forecast - by Procedure
- 5.2.1. Treatment
- 5.2.1.1. Surgery
- 5.2.1.2. Immunotherapy
- 5.2.1.3. Radiation Therapy
- 5.2.1.4. Chemotherapy
- 5.2.1.5. Other Treatments
- 5.2.2. Diagnostics
- 5.2.2.1. Biopsy
- 5.2.2.2. Ultrasound
- 5.2.2.3. Hysteroscopy
- 5.2.2.4. Dilation and Curettage
- 5.2.2.5. Other Diagnostics
- 5.2.1. Treatment
- 5.3. Market Analysis, Insights and Forecast - by Geography
- 5.3.1. North America
- 5.3.1.1. United States
- 5.3.1.2. Canada
- 5.3.1.3. Mexico
- 5.3.1. North America
- 5.4. Market Analysis, Insights and Forecast - by Region
- 5.4.1. North America
- 5.1. Market Analysis, Insights and Forecast - by Cancer Type
- 6. United States North America Uterine Cancer Diagnostics & Treatment Industry Analysis, Insights and Forecast, 2019-2031
- 7. Canada North America Uterine Cancer Diagnostics & Treatment Industry Analysis, Insights and Forecast, 2019-2031
- 8. Mexico North America Uterine Cancer Diagnostics & Treatment Industry Analysis, Insights and Forecast, 2019-2031
- 9. Rest of North America North America Uterine Cancer Diagnostics & Treatment Industry Analysis, Insights and Forecast, 2019-2031
- 10. Competitive Analysis
- 10.1. Market Share Analysis 2024
- 10.2. Company Profiles
- 10.2.1 Becton Dickinson and Company
- 10.2.1.1. Overview
- 10.2.1.2. Products
- 10.2.1.3. SWOT Analysis
- 10.2.1.4. Recent Developments
- 10.2.1.5. Financials (Based on Availability)
- 10.2.2 Novartis AG
- 10.2.2.1. Overview
- 10.2.2.2. Products
- 10.2.2.3. SWOT Analysis
- 10.2.2.4. Recent Developments
- 10.2.2.5. Financials (Based on Availability)
- 10.2.3 Merck & Co Inc
- 10.2.3.1. Overview
- 10.2.3.2. Products
- 10.2.3.3. SWOT Analysis
- 10.2.3.4. Recent Developments
- 10.2.3.5. Financials (Based on Availability)
- 10.2.4 F Hoffmann-La Roche Ltd
- 10.2.4.1. Overview
- 10.2.4.2. Products
- 10.2.4.3. SWOT Analysis
- 10.2.4.4. Recent Developments
- 10.2.4.5. Financials (Based on Availability)
- 10.2.5 AbbVie Inc
- 10.2.5.1. Overview
- 10.2.5.2. Products
- 10.2.5.3. SWOT Analysis
- 10.2.5.4. Recent Developments
- 10.2.5.5. Financials (Based on Availability)
- 10.2.6 Takeda Pharmaceutical Company Limited*List Not Exhaustive
- 10.2.6.1. Overview
- 10.2.6.2. Products
- 10.2.6.3. SWOT Analysis
- 10.2.6.4. Recent Developments
- 10.2.6.5. Financials (Based on Availability)
- 10.2.7 Bristol-Myers Squibb Company
- 10.2.7.1. Overview
- 10.2.7.2. Products
- 10.2.7.3. SWOT Analysis
- 10.2.7.4. Recent Developments
- 10.2.7.5. Financials (Based on Availability)
- 10.2.8 GlaxoSmithKline PLC
- 10.2.8.1. Overview
- 10.2.8.2. Products
- 10.2.8.3. SWOT Analysis
- 10.2.8.4. Recent Developments
- 10.2.8.5. Financials (Based on Availability)
- 10.2.9 Pfizer Inc
- 10.2.9.1. Overview
- 10.2.9.2. Products
- 10.2.9.3. SWOT Analysis
- 10.2.9.4. Recent Developments
- 10.2.9.5. Financials (Based on Availability)
- 10.2.1 Becton Dickinson and Company
List of Figures
- Figure 1: North America Uterine Cancer Diagnostics & Treatment Industry Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: North America Uterine Cancer Diagnostics & Treatment Industry Share (%) by Company 2024
List of Tables
- Table 1: North America Uterine Cancer Diagnostics & Treatment Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 2: North America Uterine Cancer Diagnostics & Treatment Industry Revenue Million Forecast, by Cancer Type 2019 & 2032
- Table 3: North America Uterine Cancer Diagnostics & Treatment Industry Revenue Million Forecast, by Procedure 2019 & 2032
- Table 4: North America Uterine Cancer Diagnostics & Treatment Industry Revenue Million Forecast, by Geography 2019 & 2032
- Table 5: North America Uterine Cancer Diagnostics & Treatment Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 6: North America Uterine Cancer Diagnostics & Treatment Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 7: United States North America Uterine Cancer Diagnostics & Treatment Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 8: Canada North America Uterine Cancer Diagnostics & Treatment Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 9: Mexico North America Uterine Cancer Diagnostics & Treatment Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 10: Rest of North America North America Uterine Cancer Diagnostics & Treatment Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 11: North America Uterine Cancer Diagnostics & Treatment Industry Revenue Million Forecast, by Cancer Type 2019 & 2032
- Table 12: North America Uterine Cancer Diagnostics & Treatment Industry Revenue Million Forecast, by Procedure 2019 & 2032
- Table 13: North America Uterine Cancer Diagnostics & Treatment Industry Revenue Million Forecast, by Geography 2019 & 2032
- Table 14: North America Uterine Cancer Diagnostics & Treatment Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 15: United States North America Uterine Cancer Diagnostics & Treatment Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 16: Canada North America Uterine Cancer Diagnostics & Treatment Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 17: Mexico North America Uterine Cancer Diagnostics & Treatment Industry Revenue (Million) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the North America Uterine Cancer Diagnostics & Treatment Industry?
The projected CAGR is approximately 4.22%.
2. Which companies are prominent players in the North America Uterine Cancer Diagnostics & Treatment Industry?
Key companies in the market include Becton Dickinson and Company, Novartis AG, Merck & Co Inc, F Hoffmann-La Roche Ltd, AbbVie Inc, Takeda Pharmaceutical Company Limited*List Not Exhaustive, Bristol-Myers Squibb Company, GlaxoSmithKline PLC, Pfizer Inc.
3. What are the main segments of the North America Uterine Cancer Diagnostics & Treatment Industry?
The market segments include Cancer Type, Procedure, Geography.
4. Can you provide details about the market size?
The market size is estimated to be USD 9.57 Million as of 2022.
5. What are some drivers contributing to market growth?
Rising Awareness about Uterine Diseases and the Available Therapies; Increasing Health Care Expenditure; Innovation in Drug Development and Subsequent Technological Advancements.
6. What are the notable trends driving market growth?
Immunotherapy Segment is Expected to Register Considerable Growth Over the Forecast Period.
7. Are there any restraints impacting market growth?
Low Success Rate in Clinical Trials for Cancer Drugs and High Cost of Research and Development; High Cost Associated with the Treatment.
8. Can you provide examples of recent developments in the market?
February 2023: GSK announced that the United States Food and Drug Administration (FDA) has granted full approval for Jemperli (dostarlimab-gxly) for the treatment of adult patients with recurrent or advanced mismatch repair-deficient endometrial cancer (dMMR) as determined by the United States FDA.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "North America Uterine Cancer Diagnostics & Treatment Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the North America Uterine Cancer Diagnostics & Treatment Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the North America Uterine Cancer Diagnostics & Treatment Industry?
To stay informed about further developments, trends, and reports in the North America Uterine Cancer Diagnostics & Treatment Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


