Key Insights
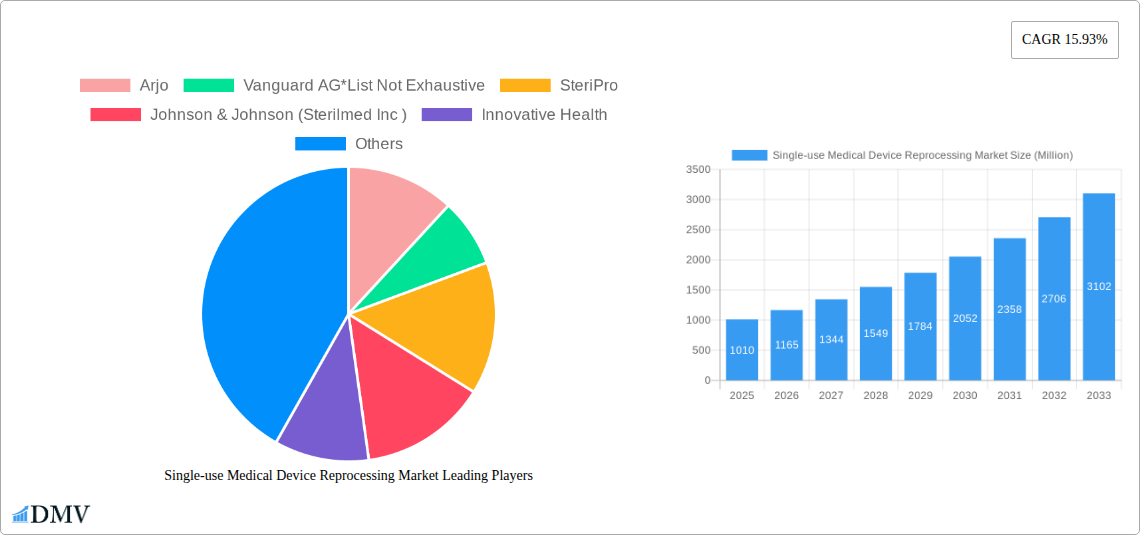
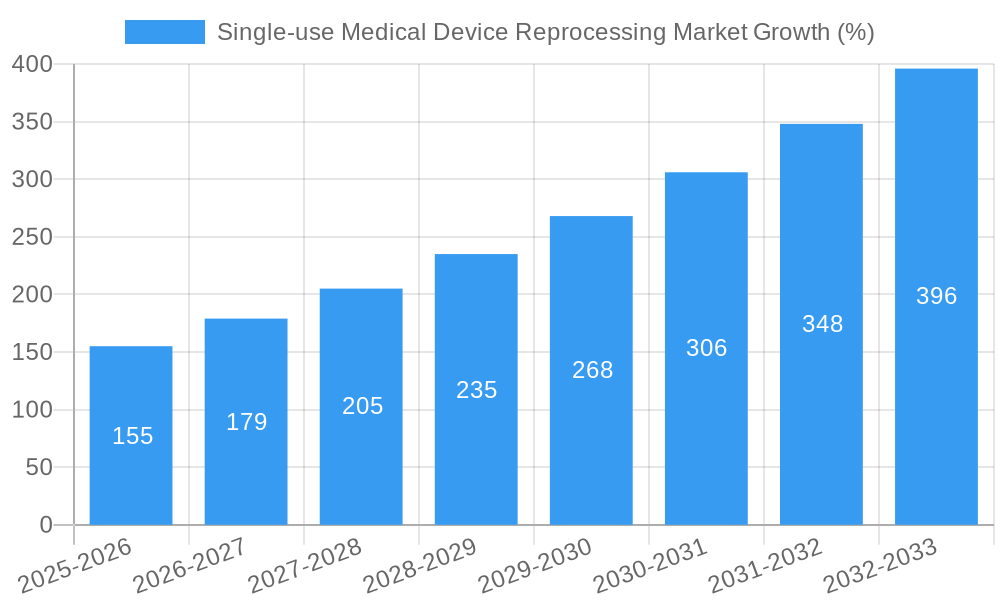
The Single-use Medical Device Reprocessing (SUDR) market is experiencing robust growth, projected to reach $1.01 billion in 2025 and exhibiting a Compound Annual Growth Rate (CAGR) of 15.93% from 2025 to 2033. This expansion is fueled by several key factors. Increasing healthcare costs are driving the need for cost-effective alternatives to single-use devices, making reprocessing an attractive option. Simultaneously, heightened awareness of environmental sustainability and the generation of substantial medical waste are prompting healthcare facilities to adopt more eco-friendly practices. Technological advancements in reprocessing techniques are also contributing to the market's growth, leading to improved safety and efficacy. The market is segmented by device type, with Class I and Class II devices representing significant portions. North America, particularly the United States, currently dominates the market, reflecting high adoption rates and advanced healthcare infrastructure. However, other regions like Asia-Pacific are witnessing rapid growth due to increasing disposable incomes and improving healthcare facilities. Competitive dynamics involve established players like Johnson & Johnson and Stryker, alongside smaller, specialized companies focusing on innovative reprocessing technologies.
The future of the SUDR market hinges on regulatory approvals for new technologies and the continued development of robust, standardized reprocessing protocols. Addressing potential restraints, such as concerns about the sterility of reprocessed devices and the need for skilled personnel to operate sophisticated equipment, will be crucial for sustainable market expansion. The market’s potential is considerable, particularly in emerging economies, where the demand for affordable healthcare is high. The continued focus on improving efficiency and safety, combined with a greater emphasis on environmental responsibility, will likely solidify the long-term growth trajectory of the SUDR market.
Single-Use Medical Device Reprocessing Market: A Comprehensive Report (2019-2033)
This insightful report provides a comprehensive analysis of the Single-Use Medical Device Reprocessing market, offering a detailed examination of market trends, growth drivers, challenges, and future opportunities. The study period spans from 2019 to 2033, with 2025 serving as both the base and estimated year. The report utilizes a robust methodology to forecast market dynamics from 2025 to 2033, providing critical insights for stakeholders across the medical device industry. The market is expected to reach xx Million by 2033.
Single-use Medical Device Reprocessing Market Market Composition & Trends
This section delves into the intricate dynamics of the single-use medical device reprocessing market, examining market concentration, innovation, regulations, substitutes, end-users, and M&A activity. The market exhibits a moderately fragmented landscape, with key players such as Arjo, Vanguard AG, SteriPro, Johnson & Johnson (Sterilmed Inc), Innovative Health, Stryker Corporation, NEScientific Inc, SureTek Medical, and Medline Industries Inc. holding significant, but not dominant, market share. Precise market share distribution is challenging to obtain due to the private nature of some company data, but estimates suggest a top 5 players control approximately xx% of the market. M&A activity has been relatively low in recent years, with deal values averaging around xx Million per transaction. Innovation is driven by the need for cost-effective solutions and improved sterilization techniques. Regulatory landscapes vary considerably across different jurisdictions, influencing adoption rates. Substitute products, such as disposable devices, compete on convenience, though the growing emphasis on sustainability and cost-effectiveness is increasingly favoring reprocessing. End-users primarily comprise hospitals and ambulatory surgical centers, with a growing interest from office-based labs.
- Market Concentration: Moderately fragmented, with no single dominant player.
- Innovation Catalysts: Cost reduction, enhanced sterilization, sustainability concerns.
- Regulatory Landscape: Varies significantly by region, impacting adoption.
- Substitute Products: Disposable devices offer convenience but increased costs.
- End-User Profile: Hospitals, ambulatory surgical centers, office-based labs.
- M&A Activity: Relatively low, with average deal values around xx Million.
Single-use Medical Device Reproprocessing Market Industry Evolution
The single-use medical device reprocessing market has witnessed significant evolution, driven by factors such as increasing healthcare costs, growing environmental concerns, and technological advancements. From 2019 to 2024, the market experienced a Compound Annual Growth Rate (CAGR) of approximately xx%, fueled primarily by rising adoption rates in cost-conscious healthcare settings. Technological advancements, such as improved sterilization methods and automation, are streamlining the reprocessing workflow, enhancing efficiency, and increasing the acceptance of reprocessed devices. Consumer demand is shifting towards sustainable and cost-effective alternatives to single-use disposable devices, creating a favorable market environment for reprocessing. This trend is further amplified by growing regulatory support and industry initiatives aimed at promoting responsible resource management. The forecast period (2025-2033) predicts a CAGR of xx%, driven by continued technological progress and expanding market adoption across various regions.
Leading Regions, Countries, or Segments in Single-use Medical Device Reprocessing Market
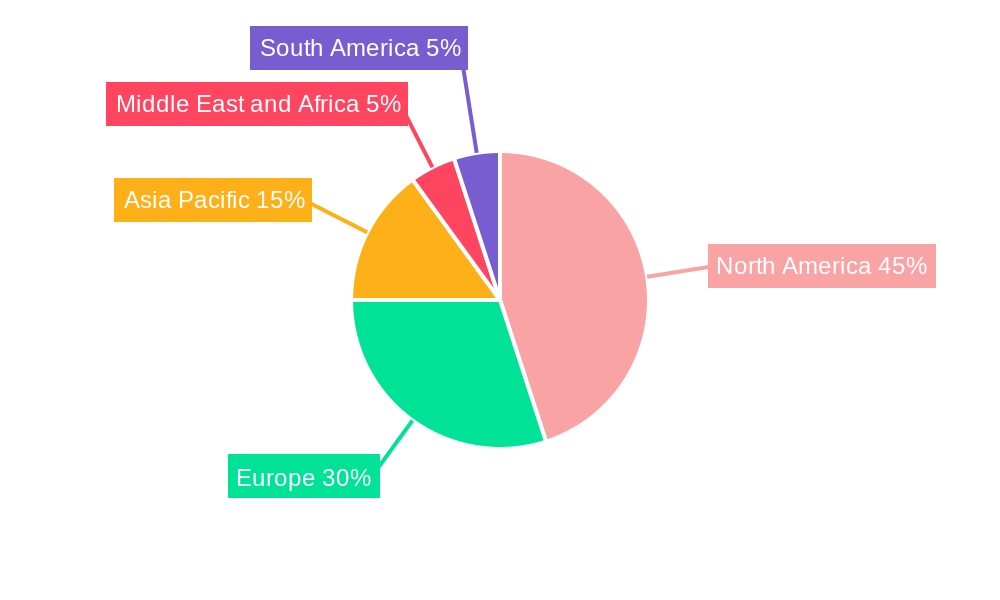
The North American market currently dominates the single-use medical device reprocessing landscape, driven by strong regulatory support, high healthcare expenditure, and a significant installed base of medical devices. Within North America, the United States holds a substantial share due to its advanced healthcare infrastructure and the presence of key market players.
- Key Drivers in North America:
- High healthcare expenditure and investment in advanced technologies.
- Stringent regulatory frameworks ensuring safety and efficacy.
- Presence of established market players and robust healthcare infrastructure.
- Growing adoption of reprocessing methods in hospitals and clinics.
The European market follows closely behind, exhibiting strong growth potential due to increasing environmental consciousness and government initiatives promoting sustainable healthcare practices. The Class II devices segment exhibits higher growth due to their increased complexity and consequent higher reprocessing costs savings.
- Factors Contributing to North American Dominance:
- High healthcare expenditure: the substantial spending on healthcare provides a strong financial incentive for adopting cost-saving practices like device reprocessing.
- Technological advancement: North America has consistently been at the forefront of medical technology development, resulting in a wider range of reprocessable devices.
- Regulatory support: favorable regulatory frameworks that ensure the safety and efficacy of reprocessed devices create trust amongst healthcare providers.
- Strong market players: the presence of major players within North America contributes to the market's competitiveness and overall growth.
Single-use Medical Device Reprocessing Market Product Innovations
Recent innovations in single-use medical device reprocessing focus on improving sterilization techniques, automation, and traceability. Advanced sterilization technologies, such as automated cleaning and disinfection systems, ensure thorough removal of contaminants and enhance safety. Integrated tracking and monitoring systems improve process efficiency and provide greater transparency throughout the reprocessing lifecycle. These innovations enhance the reliability and cost-effectiveness of reprocessing, making it a more attractive option for healthcare providers. The development of specialized reprocessing protocols for specific device types further addresses safety concerns and increases the range of devices suitable for reprocessing.
Propelling Factors for Single-use Medical Device Reprocessing Market Growth
Several factors propel growth in the single-use medical device reprocessing market. Firstly, the escalating cost of disposable medical devices is forcing healthcare providers to seek cost-effective alternatives. Secondly, the growing awareness of environmental sustainability is pushing for responsible waste management practices. Thirdly, technological advancements in sterilization and automation have improved the safety and efficiency of reprocessing. Finally, supportive regulatory frameworks are encouraging broader adoption. For instance, the FDA's 510(k) clearance for reprocessing specific devices demonstrates regulatory recognition of the safety and efficacy of this practice.
Obstacles in the Single-use Medical Device Reprocessing Market Market
Despite the significant growth potential, the single-use medical device reprocessing market faces several obstacles. Strict regulatory requirements for device reprocessing add complexity and increase costs, while establishing trust and ensuring device safety remains a concern. Supply chain disruptions, particularly regarding specialized cleaning and sterilization agents, can impact reprocessing capacity. The robust competition from manufacturers of disposable medical devices presents a persistent challenge. These factors, among others, create a complex and ever-evolving market.
Future Opportunities in Single-use Medical Device Reprocessing Market
Future opportunities in the single-use medical device reprocessing market include expanding into emerging markets with increasing healthcare infrastructure, developing advanced reprocessing technologies for complex medical devices, and promoting eco-friendly practices through robust sustainability initiatives. Collaborations between manufacturers, regulatory bodies, and healthcare providers are vital for facilitating market expansion and addressing remaining challenges.
Major Players in the Single-use Medical Device Reprocessing Market Ecosystem
- Arjo
- Vanguard AG
- SteriPro
- Johnson & Johnson (Sterilmed Inc)
- Innovative Health
- Stryker Corporation
- NEScientific Inc
- SureTek Medical
- Medline Industries Inc
Key Developments in Single-use Medical Device Reprocessing Market Industry
- February 2023: Northeast Scientific Inc. received FDA 510(k) clearance for reprocessing the Philips IVUS Eagle Eye Platinum RX Digital catheter, leading to potential cost savings for US healthcare facilities.
- June 2022: The Association of Medical Device Reprocessors launched 'Global Regulatory Standards for 'Single-Use' Medical Device Reprocessing and Remanufacturing,' providing a roadmap for regulatory bodies worldwide.
Strategic Single-use Medical Device Reprocessing Market Market Forecast
The single-use medical device reprocessing market is poised for substantial growth driven by escalating healthcare costs, environmental awareness, and technological advancements. Future opportunities lie in expanding into new geographical markets, developing innovative reprocessing technologies, and strengthening partnerships across the value chain. The market's growth trajectory is projected to remain positive, driven by the increasing adoption of sustainable and cost-effective healthcare practices.
Single-use Medical Device Reprocessing Market Segmentation
-
1. Device Type
-
1.1. Class I Devices
- 1.1.1. Laparoscopic Graspers
- 1.1.2. Scalpels
- 1.1.3. Tourniquet Cuffs
- 1.1.4. Other Class I Devices
-
1.2. Class II Devices
- 1.2.1. Pulse Oximeter Sensors
- 1.2.2. Sequential Compression Sleeves
- 1.2.3. Catheters and Guidewires
- 1.2.4. Other Class II Devices
-
1.1. Class I Devices
Single-use Medical Device Reprocessing Market Segmentation By Geography
-
1. North America
- 1.1. United States
- 1.2. Canada
- 1.3. Mexico
-
2. Europe
- 2.1. Germany
- 2.2. United Kingdom
- 2.3. France
- 2.4. Italy
- 2.5. Spain
- 2.6. Rest of Europe
-
3. Asia Pacific
- 3.1. China
- 3.2. Japan
- 3.3. India
- 3.4. Australia
- 3.5. South Korea
- 3.6. Rest of Asia Pacific
-
4. Middle East and Africa
- 4.1. GCC
- 4.2. South Africa
- 4.3. Rest of Middle East and Africa
-
5. South America
- 5.1. Brazil
- 5.2. Argentina
- 5.3. Rest of South America
Single-use Medical Device Reprocessing Market REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 15.93% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Cost Savings Through Reprocessing Single-use Devices; Regulatory Pressure to Reduce Volume of Medical Waste
- 3.3. Market Restrains
- 3.3.1. Potential of Material Alteration and Cross Infection with Reprocessed Device; Preconceived Notions Regarding the Quality of Reprocessed Single-use Medical Devices (SUDs)
- 3.4. Market Trends
- 3.4.1. Sequential Compression Sleeves Segment is Expected to Hold a Significant Market Share Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Global Single-use Medical Device Reprocessing Market Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 5.1.1. Class I Devices
- 5.1.1.1. Laparoscopic Graspers
- 5.1.1.2. Scalpels
- 5.1.1.3. Tourniquet Cuffs
- 5.1.1.4. Other Class I Devices
- 5.1.2. Class II Devices
- 5.1.2.1. Pulse Oximeter Sensors
- 5.1.2.2. Sequential Compression Sleeves
- 5.1.2.3. Catheters and Guidewires
- 5.1.2.4. Other Class II Devices
- 5.1.1. Class I Devices
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. North America
- 5.2.2. Europe
- 5.2.3. Asia Pacific
- 5.2.4. Middle East and Africa
- 5.2.5. South America
- 5.1. Market Analysis, Insights and Forecast - by Device Type
- 6. North America Single-use Medical Device Reprocessing Market Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Device Type
- 6.1.1. Class I Devices
- 6.1.1.1. Laparoscopic Graspers
- 6.1.1.2. Scalpels
- 6.1.1.3. Tourniquet Cuffs
- 6.1.1.4. Other Class I Devices
- 6.1.2. Class II Devices
- 6.1.2.1. Pulse Oximeter Sensors
- 6.1.2.2. Sequential Compression Sleeves
- 6.1.2.3. Catheters and Guidewires
- 6.1.2.4. Other Class II Devices
- 6.1.1. Class I Devices
- 6.1. Market Analysis, Insights and Forecast - by Device Type
- 7. Europe Single-use Medical Device Reprocessing Market Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Device Type
- 7.1.1. Class I Devices
- 7.1.1.1. Laparoscopic Graspers
- 7.1.1.2. Scalpels
- 7.1.1.3. Tourniquet Cuffs
- 7.1.1.4. Other Class I Devices
- 7.1.2. Class II Devices
- 7.1.2.1. Pulse Oximeter Sensors
- 7.1.2.2. Sequential Compression Sleeves
- 7.1.2.3. Catheters and Guidewires
- 7.1.2.4. Other Class II Devices
- 7.1.1. Class I Devices
- 7.1. Market Analysis, Insights and Forecast - by Device Type
- 8. Asia Pacific Single-use Medical Device Reprocessing Market Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Device Type
- 8.1.1. Class I Devices
- 8.1.1.1. Laparoscopic Graspers
- 8.1.1.2. Scalpels
- 8.1.1.3. Tourniquet Cuffs
- 8.1.1.4. Other Class I Devices
- 8.1.2. Class II Devices
- 8.1.2.1. Pulse Oximeter Sensors
- 8.1.2.2. Sequential Compression Sleeves
- 8.1.2.3. Catheters and Guidewires
- 8.1.2.4. Other Class II Devices
- 8.1.1. Class I Devices
- 8.1. Market Analysis, Insights and Forecast - by Device Type
- 9. Middle East and Africa Single-use Medical Device Reprocessing Market Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Device Type
- 9.1.1. Class I Devices
- 9.1.1.1. Laparoscopic Graspers
- 9.1.1.2. Scalpels
- 9.1.1.3. Tourniquet Cuffs
- 9.1.1.4. Other Class I Devices
- 9.1.2. Class II Devices
- 9.1.2.1. Pulse Oximeter Sensors
- 9.1.2.2. Sequential Compression Sleeves
- 9.1.2.3. Catheters and Guidewires
- 9.1.2.4. Other Class II Devices
- 9.1.1. Class I Devices
- 9.1. Market Analysis, Insights and Forecast - by Device Type
- 10. South America Single-use Medical Device Reprocessing Market Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Device Type
- 10.1.1. Class I Devices
- 10.1.1.1. Laparoscopic Graspers
- 10.1.1.2. Scalpels
- 10.1.1.3. Tourniquet Cuffs
- 10.1.1.4. Other Class I Devices
- 10.1.2. Class II Devices
- 10.1.2.1. Pulse Oximeter Sensors
- 10.1.2.2. Sequential Compression Sleeves
- 10.1.2.3. Catheters and Guidewires
- 10.1.2.4. Other Class II Devices
- 10.1.1. Class I Devices
- 10.1. Market Analysis, Insights and Forecast - by Device Type
- 11. North America Single-use Medical Device Reprocessing Market Analysis, Insights and Forecast, 2019-2031
- 11.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 11.1.1 United States
- 11.1.2 Canada
- 11.1.3 Mexico
- 12. Europe Single-use Medical Device Reprocessing Market Analysis, Insights and Forecast, 2019-2031
- 12.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 12.1.1 Germany
- 12.1.2 United Kingdom
- 12.1.3 France
- 12.1.4 Italy
- 12.1.5 Spain
- 12.1.6 Rest of Europe
- 13. Asia Pacific Single-use Medical Device Reprocessing Market Analysis, Insights and Forecast, 2019-2031
- 13.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 13.1.1 China
- 13.1.2 Japan
- 13.1.3 India
- 13.1.4 Australia
- 13.1.5 South Korea
- 13.1.6 Rest of Asia Pacific
- 14. Middle East and Africa Single-use Medical Device Reprocessing Market Analysis, Insights and Forecast, 2019-2031
- 14.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 14.1.1 GCC
- 14.1.2 South Africa
- 14.1.3 Rest of Middle East and Africa
- 15. South America Single-use Medical Device Reprocessing Market Analysis, Insights and Forecast, 2019-2031
- 15.1. Market Analysis, Insights and Forecast - By Country/Sub-region
- 15.1.1 Brazil
- 15.1.2 Argentina
- 15.1.3 Rest of South America
- 16. Competitive Analysis
- 16.1. Global Market Share Analysis 2024
- 16.2. Company Profiles
- 16.2.1 Arjo
- 16.2.1.1. Overview
- 16.2.1.2. Products
- 16.2.1.3. SWOT Analysis
- 16.2.1.4. Recent Developments
- 16.2.1.5. Financials (Based on Availability)
- 16.2.2 Vanguard AG*List Not Exhaustive
- 16.2.2.1. Overview
- 16.2.2.2. Products
- 16.2.2.3. SWOT Analysis
- 16.2.2.4. Recent Developments
- 16.2.2.5. Financials (Based on Availability)
- 16.2.3 SteriPro
- 16.2.3.1. Overview
- 16.2.3.2. Products
- 16.2.3.3. SWOT Analysis
- 16.2.3.4. Recent Developments
- 16.2.3.5. Financials (Based on Availability)
- 16.2.4 Johnson & Johnson (Sterilmed Inc )
- 16.2.4.1. Overview
- 16.2.4.2. Products
- 16.2.4.3. SWOT Analysis
- 16.2.4.4. Recent Developments
- 16.2.4.5. Financials (Based on Availability)
- 16.2.5 Innovative Health
- 16.2.5.1. Overview
- 16.2.5.2. Products
- 16.2.5.3. SWOT Analysis
- 16.2.5.4. Recent Developments
- 16.2.5.5. Financials (Based on Availability)
- 16.2.6 Stryker Corporation
- 16.2.6.1. Overview
- 16.2.6.2. Products
- 16.2.6.3. SWOT Analysis
- 16.2.6.4. Recent Developments
- 16.2.6.5. Financials (Based on Availability)
- 16.2.7 NEScientific Inc
- 16.2.7.1. Overview
- 16.2.7.2. Products
- 16.2.7.3. SWOT Analysis
- 16.2.7.4. Recent Developments
- 16.2.7.5. Financials (Based on Availability)
- 16.2.8 SureTek Medical
- 16.2.8.1. Overview
- 16.2.8.2. Products
- 16.2.8.3. SWOT Analysis
- 16.2.8.4. Recent Developments
- 16.2.8.5. Financials (Based on Availability)
- 16.2.9 Medline Industries Inc
- 16.2.9.1. Overview
- 16.2.9.2. Products
- 16.2.9.3. SWOT Analysis
- 16.2.9.4. Recent Developments
- 16.2.9.5. Financials (Based on Availability)
- 16.2.1 Arjo
List of Figures
- Figure 1: Global Single-use Medical Device Reprocessing Market Revenue Breakdown (Million, %) by Region 2024 & 2032
- Figure 2: North America Single-use Medical Device Reprocessing Market Revenue (Million), by Country 2024 & 2032
- Figure 3: North America Single-use Medical Device Reprocessing Market Revenue Share (%), by Country 2024 & 2032
- Figure 4: Europe Single-use Medical Device Reprocessing Market Revenue (Million), by Country 2024 & 2032
- Figure 5: Europe Single-use Medical Device Reprocessing Market Revenue Share (%), by Country 2024 & 2032
- Figure 6: Asia Pacific Single-use Medical Device Reprocessing Market Revenue (Million), by Country 2024 & 2032
- Figure 7: Asia Pacific Single-use Medical Device Reprocessing Market Revenue Share (%), by Country 2024 & 2032
- Figure 8: Middle East and Africa Single-use Medical Device Reprocessing Market Revenue (Million), by Country 2024 & 2032
- Figure 9: Middle East and Africa Single-use Medical Device Reprocessing Market Revenue Share (%), by Country 2024 & 2032
- Figure 10: South America Single-use Medical Device Reprocessing Market Revenue (Million), by Country 2024 & 2032
- Figure 11: South America Single-use Medical Device Reprocessing Market Revenue Share (%), by Country 2024 & 2032
- Figure 12: North America Single-use Medical Device Reprocessing Market Revenue (Million), by Device Type 2024 & 2032
- Figure 13: North America Single-use Medical Device Reprocessing Market Revenue Share (%), by Device Type 2024 & 2032
- Figure 14: North America Single-use Medical Device Reprocessing Market Revenue (Million), by Country 2024 & 2032
- Figure 15: North America Single-use Medical Device Reprocessing Market Revenue Share (%), by Country 2024 & 2032
- Figure 16: Europe Single-use Medical Device Reprocessing Market Revenue (Million), by Device Type 2024 & 2032
- Figure 17: Europe Single-use Medical Device Reprocessing Market Revenue Share (%), by Device Type 2024 & 2032
- Figure 18: Europe Single-use Medical Device Reprocessing Market Revenue (Million), by Country 2024 & 2032
- Figure 19: Europe Single-use Medical Device Reprocessing Market Revenue Share (%), by Country 2024 & 2032
- Figure 20: Asia Pacific Single-use Medical Device Reprocessing Market Revenue (Million), by Device Type 2024 & 2032
- Figure 21: Asia Pacific Single-use Medical Device Reprocessing Market Revenue Share (%), by Device Type 2024 & 2032
- Figure 22: Asia Pacific Single-use Medical Device Reprocessing Market Revenue (Million), by Country 2024 & 2032
- Figure 23: Asia Pacific Single-use Medical Device Reprocessing Market Revenue Share (%), by Country 2024 & 2032
- Figure 24: Middle East and Africa Single-use Medical Device Reprocessing Market Revenue (Million), by Device Type 2024 & 2032
- Figure 25: Middle East and Africa Single-use Medical Device Reprocessing Market Revenue Share (%), by Device Type 2024 & 2032
- Figure 26: Middle East and Africa Single-use Medical Device Reprocessing Market Revenue (Million), by Country 2024 & 2032
- Figure 27: Middle East and Africa Single-use Medical Device Reprocessing Market Revenue Share (%), by Country 2024 & 2032
- Figure 28: South America Single-use Medical Device Reprocessing Market Revenue (Million), by Device Type 2024 & 2032
- Figure 29: South America Single-use Medical Device Reprocessing Market Revenue Share (%), by Device Type 2024 & 2032
- Figure 30: South America Single-use Medical Device Reprocessing Market Revenue (Million), by Country 2024 & 2032
- Figure 31: South America Single-use Medical Device Reprocessing Market Revenue Share (%), by Country 2024 & 2032
List of Tables
- Table 1: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Device Type 2019 & 2032
- Table 3: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Region 2019 & 2032
- Table 4: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 5: United States Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 6: Canada Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 7: Mexico Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 8: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 9: Germany Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 10: United Kingdom Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 11: France Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 12: Italy Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 13: Spain Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 14: Rest of Europe Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 15: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 16: China Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 17: Japan Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 18: India Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 19: Australia Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 20: South Korea Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 21: Rest of Asia Pacific Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 22: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 23: GCC Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 24: South Africa Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 25: Rest of Middle East and Africa Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 26: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 27: Brazil Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 28: Argentina Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 29: Rest of South America Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 30: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Device Type 2019 & 2032
- Table 31: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 32: United States Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 33: Canada Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 34: Mexico Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 35: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Device Type 2019 & 2032
- Table 36: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 37: Germany Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 38: United Kingdom Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 39: France Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 40: Italy Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 41: Spain Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 42: Rest of Europe Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 43: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Device Type 2019 & 2032
- Table 44: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 45: China Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 46: Japan Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 47: India Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 48: Australia Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 49: South Korea Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 50: Rest of Asia Pacific Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 51: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Device Type 2019 & 2032
- Table 52: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 53: GCC Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 54: South Africa Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 55: Rest of Middle East and Africa Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 56: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Device Type 2019 & 2032
- Table 57: Global Single-use Medical Device Reprocessing Market Revenue Million Forecast, by Country 2019 & 2032
- Table 58: Brazil Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 59: Argentina Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
- Table 60: Rest of South America Single-use Medical Device Reprocessing Market Revenue (Million) Forecast, by Application 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Single-use Medical Device Reprocessing Market?
The projected CAGR is approximately 15.93%.
2. Which companies are prominent players in the Single-use Medical Device Reprocessing Market?
Key companies in the market include Arjo, Vanguard AG*List Not Exhaustive, SteriPro, Johnson & Johnson (Sterilmed Inc ), Innovative Health, Stryker Corporation, NEScientific Inc, SureTek Medical, Medline Industries Inc.
3. What are the main segments of the Single-use Medical Device Reprocessing Market?
The market segments include Device Type.
4. Can you provide details about the market size?
The market size is estimated to be USD 1.01 Million as of 2022.
5. What are some drivers contributing to market growth?
Cost Savings Through Reprocessing Single-use Devices; Regulatory Pressure to Reduce Volume of Medical Waste.
6. What are the notable trends driving market growth?
Sequential Compression Sleeves Segment is Expected to Hold a Significant Market Share Over the Forecast Period.
7. Are there any restraints impacting market growth?
Potential of Material Alteration and Cross Infection with Reprocessed Device; Preconceived Notions Regarding the Quality of Reprocessed Single-use Medical Devices (SUDs).
8. Can you provide examples of recent developments in the market?
February 2023: Northeast Scientific Inc. received FDA 510(k) clearance for reprocessing the Philips IVUS Eagle Eye Platinum RX Digital catheter. With the launch of this product, considerable savings to Office-Based Labs and Hospital Cath Labs across the United States is likely to be achieved.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Single-use Medical Device Reprocessing Market," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Single-use Medical Device Reprocessing Market report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Single-use Medical Device Reprocessing Market?
To stay informed about further developments, trends, and reports in the Single-use Medical Device Reprocessing Market, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


