Key Insights
The Saudi Arabian respiratory devices market, valued at approximately $150 million in 2025, is projected to experience robust growth, driven by a rising prevalence of chronic respiratory diseases like asthma and COPD, coupled with an aging population. The increasing healthcare expenditure in the Kingdom and government initiatives focused on improving healthcare infrastructure further fuel market expansion. Technological advancements, particularly in the development of smart inhalers and connected devices for remote patient monitoring, are transforming the landscape, offering enhanced convenience and improved disease management. The market is segmented by device type into diagnostics and monitoring equipment, therapeutic devices (e.g., ventilators, nebulizers), and disposables (e.g., oxygen masks, cannulas). While the diagnostic and therapeutic segments hold significant market share currently, the disposables segment is expected to experience relatively faster growth due to the ongoing need for consumables in respiratory care. The competitive landscape includes both international players like ResMed, Philips, and Medtronic, and local distributors. Challenges include the relatively high cost of advanced respiratory devices, which could impact accessibility in certain segments of the population.
Looking ahead to 2033, the market is anticipated to reach approximately $250 million, exhibiting a compound annual growth rate (CAGR) of 5.5%. This growth trajectory is influenced by several factors, including increased awareness of respiratory health, improved access to healthcare, and the growing adoption of innovative treatment strategies. The market's regional segmentation reflects the diverse healthcare needs across the country, with potentially higher demand in urban areas. Future market performance hinges on factors such as government healthcare policies, the pace of technological innovation, and the success of initiatives aimed at increasing public health awareness and improving early diagnosis and treatment of respiratory illnesses. Further growth is also dependent on the continued expansion of private healthcare investment and the broader economic development of the Kingdom.
This insightful report provides a detailed analysis of the Saudi Arabia respiratory devices industry, offering crucial market intelligence for stakeholders. From market size and segmentation to key players and future opportunities, this comprehensive study covers all aspects of this rapidly evolving sector. The report utilizes data from the historical period (2019-2024), the base year (2025), and provides estimations for 2025 and forecasts until 2033. The Saudi Arabian respiratory devices market is expected to reach xx Million by 2033, driven by factors such as increasing prevalence of respiratory diseases and rising healthcare expenditure.
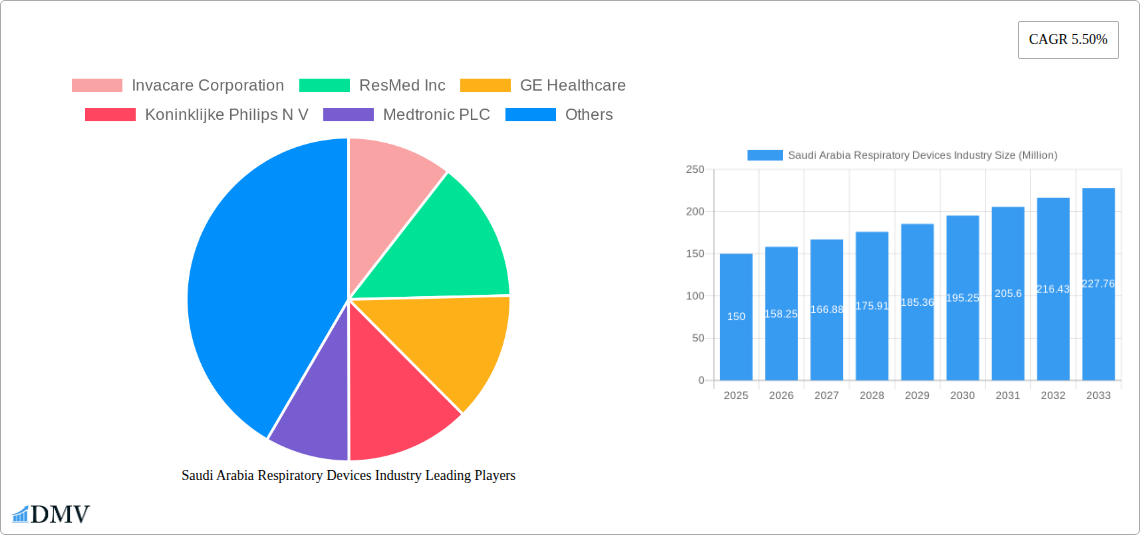
Saudi Arabia Respiratory Devices Industry Market Composition & Trends
This section delves into the competitive landscape of the Saudi Arabia respiratory devices market, examining market concentration, innovation drivers, regulatory frameworks, and substitute products. We analyze the interplay of these factors with end-user profiles and M&A activities to provide a holistic view of market dynamics. The market share distribution amongst key players—including Invacare Corporation, ResMed Inc, GE Healthcare, Koninklijke Philips N V, Medtronic PLC, Fisher & Paykel Healthcare Limited, Drägerwerk AG, and GlaxoSmithKline PLC—will be detailed. We analyze M&A activity through specific deal examples and their impact on the overall market valuation, estimating the total value of these deals at xx Million during the study period. The report further explores the influence of regulatory bodies and their impact on market access and innovation within the Saudi Arabian healthcare sector. Finally, the report assesses the impact of substitute products and explores potential disruptions from innovative technologies or treatment approaches.
- Market Concentration: Analysis of market share distribution among major players.
- Innovation Catalysts: Identification of key factors driving innovation within the industry.
- Regulatory Landscape: Detailed assessment of the regulatory environment and its influence on market growth.
- Substitute Products: Evaluation of substitute products and their potential impact on market demand.
- End-User Profiles: Profiling of key end-users, including hospitals, clinics, and home healthcare providers.
- M&A Activities: Analysis of mergers and acquisitions, including deal values and their implications.
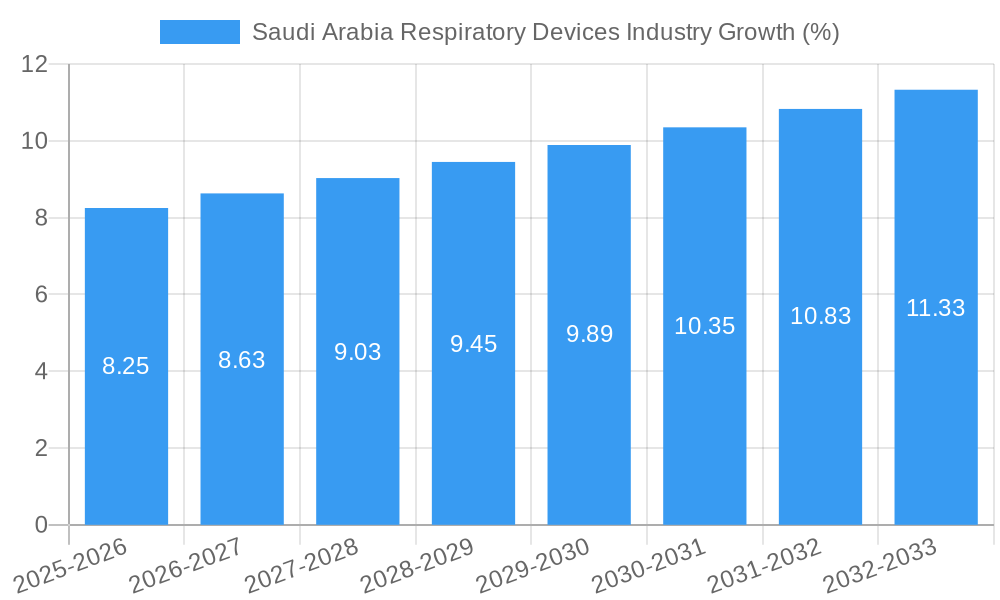
Saudi Arabia Respiratory Devices Industry Industry Evolution
This section presents a comprehensive analysis of the evolution of the Saudi Arabia respiratory devices market. We examine market growth trajectories from 2019 to 2024, projecting future growth rates for 2025-2033. This section will detail the technological advancements that have shaped the industry, including the integration of digital health solutions, the development of sophisticated diagnostic and therapeutic devices, and the shift towards minimally invasive procedures. We will also analyze shifting consumer demands, such as the growing preference for home-based respiratory care and the rising awareness of respiratory health issues. Specific data points, including compound annual growth rates (CAGR) and adoption metrics for key technologies, will be presented, highlighting trends and significant milestones. Furthermore, the influence of government initiatives and healthcare policies on market growth will be thoroughly investigated.
Leading Regions, Countries, or Segments in Saudi Arabia Respiratory Devices Industry
This section identifies the dominant regions, countries, or segments within the Saudi Arabia respiratory devices market. Focusing on the "By Type" segmentation (Diagnostic and Monitoring Devices, Therapeutic Devices, Disposables), we will determine the leading segment and provide a detailed analysis of the factors contributing to its dominance.
- Diagnostic and Monitoring Devices: Key drivers for this segment's growth in Saudi Arabia will be examined.
- Therapeutic Devices: Analysis of market dominance and factors impacting growth in this area.
- Disposables: Evaluation of the market share and key factors influencing its performance.
The dominant segment will be clearly identified with a deep dive into its market size, growth trajectory and future projections. Factors such as investment trends, regulatory support, and technological advancements will be thoroughly examined to provide a robust understanding of the segment's leading position.
Saudi Arabia Respiratory Devices Industry Product Innovations
This section highlights recent product innovations in the Saudi Arabia respiratory devices market, emphasizing their unique selling propositions and technological advancements. The report will focus on novel devices, software solutions, and materials that are enhancing the diagnosis, treatment, and management of respiratory diseases. Specific examples of innovative products will be discussed, accompanied by an analysis of their performance metrics and market impact. This section will also include a discussion of the technological advancements driving the industry's product innovation landscape.
Propelling Factors for Saudi Arabia Respiratory Devices Industry Growth
Several key factors drive the growth of the Saudi Arabia respiratory devices market. These include: the rising prevalence of chronic respiratory illnesses like asthma and COPD, increasing healthcare expenditure, government initiatives promoting preventative healthcare, and the adoption of advanced technologies in respiratory care. The growing geriatric population further fuels market expansion. Furthermore, technological advancements such as the development of smart inhalers and connected devices play a significant role.
Obstacles in the Saudi Arabia Respiratory Devices Industry Market
Despite promising growth prospects, several obstacles hinder the market's expansion. These include the high cost of advanced respiratory devices, which can limit access, particularly in underserved populations. Stringent regulatory approvals and lengthy processes can delay product launches. Supply chain disruptions, potentially impacting the availability of essential components and devices, pose another challenge. Finally, intense competition amongst major market players adds to the complexity of the market landscape.
Future Opportunities in Saudi Arabia Respiratory Devices Industry
The Saudi Arabia respiratory devices market presents substantial opportunities for growth. The increasing adoption of telehealth and remote patient monitoring offers new avenues for expanding access to respiratory care. Technological innovation, particularly in areas such as artificial intelligence (AI) and machine learning, will significantly transform diagnostic and therapeutic approaches. Furthermore, the rising awareness of respiratory health and the increasing demand for preventive care create promising opportunities for market players.
Major Players in the Saudi Arabia Respiratory Devices Industry Ecosystem
- Invacare Corporation
- ResMed Inc
- GE Healthcare
- Koninklijke Philips N V
- Medtronic PLC
- Fisher & Paykel Healthcare Limited
- Drägerwerk AG
- GlaxoSmithKline PLC
Key Developments in Saudi Arabia Respiratory Devices Industry Industry
- March 2022: Avon Protection showcased its respiratory and head protection portfolio at the World Defense Show in Riyadh.
- February 2022: Starpharma secured an exclusive sales and distribution agreement for VIRALEZE in Saudi Arabia and other GCC countries.
Strategic Saudi Arabia Respiratory Devices Industry Market Forecast
- March 2022: Avon Protection showcased its respiratory and head protection portfolio at the World Defense Show in Riyadh.
- February 2022: Starpharma secured an exclusive sales and distribution agreement for VIRALEZE in Saudi Arabia and other GCC countries.
Strategic Saudi Arabia Respiratory Devices Industry Market Forecast
The Saudi Arabia respiratory devices market is poised for robust growth, driven by technological advancements, increasing prevalence of respiratory diseases, and supportive government initiatives. The expanding healthcare infrastructure and rising healthcare expenditure will further fuel market expansion. Significant opportunities exist for companies focusing on innovative technologies, improved access to care, and addressing the unmet needs of the Saudi Arabian population. The market is anticipated to show a strong CAGR during the forecast period (2025-2033).
Saudi Arabia Respiratory Devices Industry Segmentation
-
1. Type
-
1.1. By Diagnostic and Monitoring Devices
- 1.1.1. Spirometers
- 1.1.2. Sleep Test Devices
- 1.1.3. Peak Flow Meters
- 1.1.4. Pulse Oximeters
- 1.1.5. Capnographs
- 1.1.6. Other Diagnostic and Monitoring Devices
-
1.2. By Therapeutic Devices
- 1.2.1. CPAP Devices
- 1.2.2. BiPAP Devices
- 1.2.3. Humidifiers
- 1.2.4. Nebulizers
- 1.2.5. Oxygen Concentrators
- 1.2.6. Ventilators
- 1.2.7. Inhalers
- 1.2.8. Other Therapeutic Devices
-
1.3. By Disposables
- 1.3.1. Masks
- 1.3.2. Breathing Circuits
- 1.3.3. Other Disposables
-
1.1. By Diagnostic and Monitoring Devices
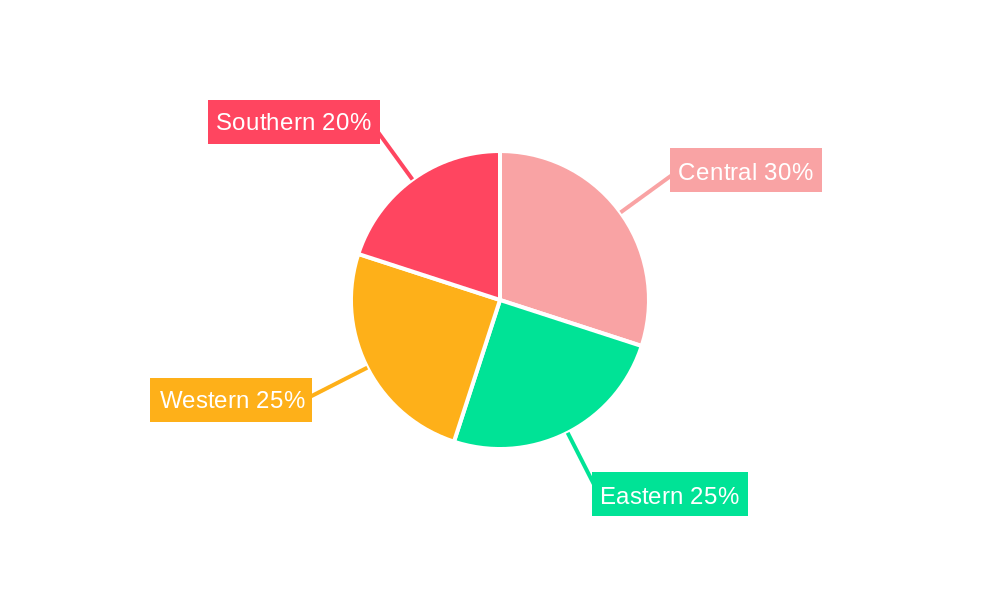
Saudi Arabia Respiratory Devices Industry Segmentation By Geography
- 1. Saudi Arabia
Saudi Arabia Respiratory Devices Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 5.50% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Prevalence of Respiratory Disorders; Rising Government Initiatives and Large Patient Population
- 3.3. Market Restrains
- 3.3.1. High Cost of Devices
- 3.4. Market Trends
- 3.4.1. Inhalers Segment is Expected to Witness Growth Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Saudi Arabia Respiratory Devices Industry Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Type
- 5.1.1. By Diagnostic and Monitoring Devices
- 5.1.1.1. Spirometers
- 5.1.1.2. Sleep Test Devices
- 5.1.1.3. Peak Flow Meters
- 5.1.1.4. Pulse Oximeters
- 5.1.1.5. Capnographs
- 5.1.1.6. Other Diagnostic and Monitoring Devices
- 5.1.2. By Therapeutic Devices
- 5.1.2.1. CPAP Devices
- 5.1.2.2. BiPAP Devices
- 5.1.2.3. Humidifiers
- 5.1.2.4. Nebulizers
- 5.1.2.5. Oxygen Concentrators
- 5.1.2.6. Ventilators
- 5.1.2.7. Inhalers
- 5.1.2.8. Other Therapeutic Devices
- 5.1.3. By Disposables
- 5.1.3.1. Masks
- 5.1.3.2. Breathing Circuits
- 5.1.3.3. Other Disposables
- 5.1.1. By Diagnostic and Monitoring Devices
- 5.2. Market Analysis, Insights and Forecast - by Region
- 5.2.1. Saudi Arabia
- 5.1. Market Analysis, Insights and Forecast - by Type
- 6. Central Saudi Arabia Respiratory Devices Industry Analysis, Insights and Forecast, 2019-2031
- 7. Eastern Saudi Arabia Respiratory Devices Industry Analysis, Insights and Forecast, 2019-2031
- 8. Western Saudi Arabia Respiratory Devices Industry Analysis, Insights and Forecast, 2019-2031
- 9. Southern Saudi Arabia Respiratory Devices Industry Analysis, Insights and Forecast, 2019-2031
- 10. Competitive Analysis
- 10.1. Market Share Analysis 2024
- 10.2. Company Profiles
- 10.2.1 Invacare Corporation
- 10.2.1.1. Overview
- 10.2.1.2. Products
- 10.2.1.3. SWOT Analysis
- 10.2.1.4. Recent Developments
- 10.2.1.5. Financials (Based on Availability)
- 10.2.2 ResMed Inc
- 10.2.2.1. Overview
- 10.2.2.2. Products
- 10.2.2.3. SWOT Analysis
- 10.2.2.4. Recent Developments
- 10.2.2.5. Financials (Based on Availability)
- 10.2.3 GE Healthcare
- 10.2.3.1. Overview
- 10.2.3.2. Products
- 10.2.3.3. SWOT Analysis
- 10.2.3.4. Recent Developments
- 10.2.3.5. Financials (Based on Availability)
- 10.2.4 Koninklijke Philips N V
- 10.2.4.1. Overview
- 10.2.4.2. Products
- 10.2.4.3. SWOT Analysis
- 10.2.4.4. Recent Developments
- 10.2.4.5. Financials (Based on Availability)
- 10.2.5 Medtronic PLC
- 10.2.5.1. Overview
- 10.2.5.2. Products
- 10.2.5.3. SWOT Analysis
- 10.2.5.4. Recent Developments
- 10.2.5.5. Financials (Based on Availability)
- 10.2.6 Fisher & Paykel Healthcare Limited
- 10.2.6.1. Overview
- 10.2.6.2. Products
- 10.2.6.3. SWOT Analysis
- 10.2.6.4. Recent Developments
- 10.2.6.5. Financials (Based on Availability)
- 10.2.7 Dragerwerk AG
- 10.2.7.1. Overview
- 10.2.7.2. Products
- 10.2.7.3. SWOT Analysis
- 10.2.7.4. Recent Developments
- 10.2.7.5. Financials (Based on Availability)
- 10.2.8 GlaxoSmithKline PLC
- 10.2.8.1. Overview
- 10.2.8.2. Products
- 10.2.8.3. SWOT Analysis
- 10.2.8.4. Recent Developments
- 10.2.8.5. Financials (Based on Availability)
- 10.2.1 Invacare Corporation
List of Figures
- Figure 1: Saudi Arabia Respiratory Devices Industry Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Saudi Arabia Respiratory Devices Industry Share (%) by Company 2024
List of Tables
- Table 1: Saudi Arabia Respiratory Devices Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Saudi Arabia Respiratory Devices Industry Volume K Unit Forecast, by Region 2019 & 2032
- Table 3: Saudi Arabia Respiratory Devices Industry Revenue Million Forecast, by Type 2019 & 2032
- Table 4: Saudi Arabia Respiratory Devices Industry Volume K Unit Forecast, by Type 2019 & 2032
- Table 5: Saudi Arabia Respiratory Devices Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 6: Saudi Arabia Respiratory Devices Industry Volume K Unit Forecast, by Region 2019 & 2032
- Table 7: Saudi Arabia Respiratory Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 8: Saudi Arabia Respiratory Devices Industry Volume K Unit Forecast, by Country 2019 & 2032
- Table 9: Central Saudi Arabia Respiratory Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 10: Central Saudi Arabia Respiratory Devices Industry Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 11: Eastern Saudi Arabia Respiratory Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 12: Eastern Saudi Arabia Respiratory Devices Industry Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 13: Western Saudi Arabia Respiratory Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 14: Western Saudi Arabia Respiratory Devices Industry Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 15: Southern Saudi Arabia Respiratory Devices Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 16: Southern Saudi Arabia Respiratory Devices Industry Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 17: Saudi Arabia Respiratory Devices Industry Revenue Million Forecast, by Type 2019 & 2032
- Table 18: Saudi Arabia Respiratory Devices Industry Volume K Unit Forecast, by Type 2019 & 2032
- Table 19: Saudi Arabia Respiratory Devices Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 20: Saudi Arabia Respiratory Devices Industry Volume K Unit Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Saudi Arabia Respiratory Devices Industry?
The projected CAGR is approximately 5.50%.
2. Which companies are prominent players in the Saudi Arabia Respiratory Devices Industry?
Key companies in the market include Invacare Corporation, ResMed Inc, GE Healthcare, Koninklijke Philips N V, Medtronic PLC, Fisher & Paykel Healthcare Limited, Dragerwerk AG, GlaxoSmithKline PLC.
3. What are the main segments of the Saudi Arabia Respiratory Devices Industry?
The market segments include Type.
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Prevalence of Respiratory Disorders; Rising Government Initiatives and Large Patient Population.
6. What are the notable trends driving market growth?
Inhalers Segment is Expected to Witness Growth Over the Forecast Period.
7. Are there any restraints impacting market growth?
High Cost of Devices.
8. Can you provide examples of recent developments in the market?
In March 2022, Avon Protection demonstrated its respiratory and head protection portfolio, including Avon Protection's FM50 respirator, MP-PAPR, and the F90 helmet at the first World Defense Show in Riyadh, Saudi Arabia.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 3800, USD 4500, and USD 5800 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Saudi Arabia Respiratory Devices Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Saudi Arabia Respiratory Devices Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Saudi Arabia Respiratory Devices Industry?
To stay informed about further developments, trends, and reports in the Saudi Arabia Respiratory Devices Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


