Key Insights
The European incontinence devices and ostomy care market is experiencing robust growth, driven by an aging population, rising prevalence of chronic diseases like bladder cancer, colorectal cancer, and Crohn's disease, and increasing awareness of available treatment options. The market, estimated at €X million in 2025, is projected to maintain a Compound Annual Growth Rate (CAGR) of 10.10% through 2033, reaching a substantial market size. This expansion is fueled by technological advancements leading to more comfortable and discreet products, improved healthcare infrastructure supporting better patient management, and increased government initiatives focused on improving the quality of life for patients with incontinence and ostomy needs. Key product segments like incontinence care products and ostomy bags (colostomy, ileostomy, and urostomy bags) contribute significantly to overall market revenue, with a slight tilt towards incontinence products based on general market trends. Germany, France, and the UK represent major market shares within Europe due to their larger populations and well-established healthcare systems.
The market's growth, however, faces certain restraints. High product costs can present a significant barrier for patients, especially those without comprehensive insurance coverage. Furthermore, the potential for adverse effects associated with certain products and the complexities of managing ostomy care require continuous improvement in product design and patient education. Competitive landscape analysis reveals key players like Unicharm, Abena, ConvaTec, Hollister, Kimberly-Clark, B. Braun, and Salts Healthcare vying for market share through product innovation, strategic partnerships, and expansion into new markets. Future growth will likely be shaped by the development of innovative materials, telehealth solutions for remote patient monitoring, and personalized care strategies tailored to individual patient needs. The focus will increasingly shift towards improving patient comfort, reducing healthcare costs, and offering comprehensive solutions that enhance the quality of life for those managing incontinence and ostomy conditions.

Europe Incontinence Devices and Ostomy Industry: A Comprehensive Market Report (2019-2033)
This insightful report provides a detailed analysis of the European incontinence devices and ostomy industry, offering a comprehensive overview of market dynamics, key players, and future growth prospects. Covering the period 2019-2033, with a base year of 2025 and a forecast period of 2025-2033, this report is essential for stakeholders seeking to understand and capitalize on opportunities within this evolving sector. The market is expected to reach xx Million by 2033.
Europe Incontinence Devices and Ostomy Industry Market Composition & Trends
The European incontinence devices and ostomy industry presents a dynamic market landscape shaped by competitive pressures, innovative technologies, stringent regulations, and evolving patient needs. This section delves into a comprehensive analysis of these key factors, providing insights into market structure, growth drivers, and future projections.
- Market Concentration and Competitive Dynamics: The market exhibits a moderately concentrated structure, with leading players such as Unicharm Corporation, Abena AS, ConvaTec, Hollister Inc, Kimberly-Clark Corporation, B Braun Melsungen AG, and Salts Healthcare holding significant market share. The report analyzes the competitive intensity, strategic alliances, and the impact of mergers and acquisitions (M&A) activities, including a quantification of deal values and their influence on market share distribution in 2024 and beyond. We project that the top five players will account for approximately [Insert Percentage]% of the total market share in 2024. Further analysis reveals the strategies employed by these key players to maintain their competitive edge.
- Innovation Catalysts: Technological advancements are revolutionizing the industry. This includes breakthroughs in absorbent materials offering enhanced comfort and performance, the integration of sensor technology for improved patient monitoring and management, and the increasing adoption of telehealth solutions enabling remote patient care and reducing healthcare costs. The report details specific examples of these innovations and their impact on market growth.
- Regulatory Landscape and Reimbursement Policies: The European regulatory environment for medical devices significantly impacts market access and product launches. This section analyzes the influence of EU regulations on market growth, highlighting the impact of reimbursement policies on patient access and market penetration. Specific regulatory hurdles and their effect on innovation and market expansion are discussed.
- Substitute Products and Treatment Options: The availability of alternative treatment strategies and management options for incontinence and ostomy care influences the overall market penetration. This section considers these alternatives and their impact on market growth, providing a thorough comparison of different approaches.
- End-User Profiles and Market Segmentation: A detailed analysis of end-user demographics, healthcare needs, and purchasing behaviors provides critical insights into market segmentation and product development strategies. This understanding of the target population is crucial for identifying unmet needs and tailoring product offerings.
- Mergers and Acquisitions (M&A) Activity: The report provides a detailed analysis of recent M&A activity in the European incontinence devices and ostomy industry, including deal values (estimated at [Insert Updated Value] Million in total for the period 2019-2024), and their impact on market consolidation and competitive landscape. We assess the strategic rationale behind these transactions and their influence on future market dynamics.
Europe Incontinence Devices and Ostomy Industry Industry Evolution
This section delves into the historical and projected growth trajectories of the European incontinence devices and ostomy industry. We analyze market size expansion, technological advancements such as the rise of digitally connected devices, and evolving consumer preferences toward improved comfort, discretion, and ease of use. The analysis incorporates specific data points on growth rates (CAGR estimated at xx% from 2025 to 2033) and adoption metrics for various product types and applications. The increasing prevalence of chronic diseases like diabetes and aging populations are key drivers fueling market expansion. The shift towards home healthcare and telehealth solutions is also examined, alongside its impact on market dynamics and consumer behavior. Further, the evolving regulatory landscape and its influence on market access and product approvals is evaluated. We forecast a significant increase in demand for advanced, high-performance incontinence and ostomy care products driven by rising healthcare expenditure and improved patient outcomes.
Leading Regions, Countries, or Segments in Europe Incontinence Devices and Ostomy Industry
This section pinpoints the leading regions, countries, and segments within the European incontinence devices and ostomy market, offering a granular analysis of market dominance based on key factors:
By Product Type:
- Incontinence Care Products: This segment commands the largest market share due to the high prevalence of urinary and fecal incontinence across various demographic groups. The report analyzes specific product categories within this segment and their respective market shares.
- Ostomy Care Products: This segment experiences robust growth fueled by increased awareness of ostomy care, improved treatment options for related conditions, and enhanced product offerings. We provide a breakdown of the various sub-segments within ostomy care.
- Ostomy Bags (Colostomy, Ileostomy, Urostomy): A detailed analysis of market share for each type of ostomy bag considers factors such as patient demographics, prevalence of related diseases, and evolving treatment protocols.
By Application:
- Bladder Cancer: The rising incidence of bladder cancer across Europe drives significant growth within this application segment. The report analyzes the specific needs and product preferences within this patient population.
- Colorectal Cancer: Similar to bladder cancer, the increasing prevalence of colorectal cancer fuels strong demand for ostomy care products. We explore the specific products and services utilized in this context.
- Crohn's Disease: The chronic nature of Crohn's disease contributes to consistent demand for ostomy care products, necessitating long-term management solutions. The report examines the implications of this chronic condition on market demand.
- Kidney Stone & Chronic Kidney Failure: These conditions drive specific needs for incontinence and ostomy management solutions. The report segments the market based on the specific products used for these conditions.
- Other Applications: This includes other conditions leading to incontinence or ostomy needs, offering a comprehensive perspective of the overall market.
Key Drivers (across segments and applications):
- Significant investments in research and development, fostering continuous innovation and product improvements.
- Favorable reimbursement policies and well-developed healthcare infrastructure in several European countries.
- Strong regulatory support for medical devices, focused on improving patient outcomes and safety.
- Growing awareness among patients and healthcare professionals about effective management strategies.
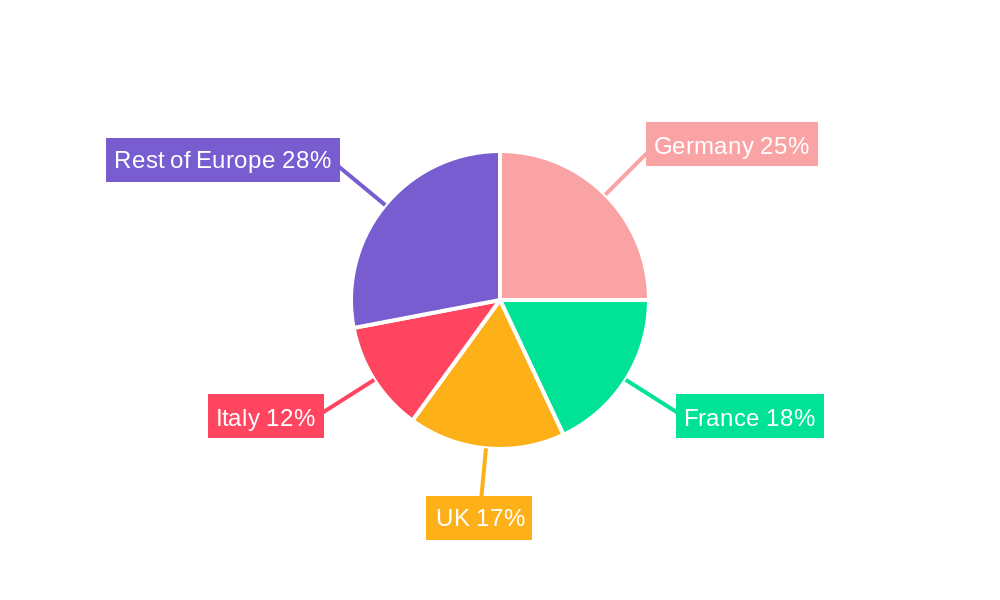
Germany, France, and the UK remain the leading countries in the European market due to factors such as higher healthcare expenditure, aging populations, and robust healthcare infrastructure. The report provides a more in-depth analysis of these key countries, including regional variations and competitive dynamics.
Europe Incontinence Devices and Ostomy Industry Product Innovations
Recent innovations focus on enhanced comfort, discretion, and ease of use. New materials are improving absorbency and reducing skin irritation. Smart sensors integrated into devices provide real-time data on usage and physiological parameters, facilitating remote patient monitoring and improved care management. The development of user-friendly designs and telehealth solutions is enhancing patient experience and care quality. Examples of this include Ontex's Orizon digital incontinence management service, launched in late 2022/early 2023.
Propelling Factors for Europe Incontinence Devices and Ostomy Industry Growth
Several factors drive the growth of the European incontinence devices and ostomy market: The increasing prevalence of chronic diseases, such as diabetes and heart failure, directly contributes to a rise in incontinence cases. An aging population leads to a greater demand for these products. Technological advancements, particularly in absorbent materials and telehealth solutions, create more effective and convenient options. Favorable reimbursement policies by government agencies and healthcare providers increase market accessibility. Finally, rising healthcare expenditure across European nations provides the financial resources to support the market's expansion.
Obstacles in the Europe Incontinence Devices and Ostomy Industry Market
Despite significant growth potential, the European incontinence devices and ostomy industry faces several challenges. These include stringent regulatory approvals for new medical devices, leading to delays in product launches and increased development costs. Supply chain disruptions and fluctuating raw material costs pose significant risks to production and profitability. Intense competition among established and emerging players exerts pressure on pricing and profit margins, requiring companies to innovate and differentiate themselves. Finally, the stringent hygiene standards associated with these products necessitate robust quality control measures and meticulous sterility protocols, adding to manufacturing costs.
Future Opportunities in Europe Incontinence Devices and Ostomy Industry
Future growth lies in expanding into underpenetrated markets, developing customized solutions for specific patient needs, and leveraging technological advancements to improve product design and user experience. The integration of telehealth and remote patient monitoring technologies has the potential to revolutionize care delivery, providing patients with improved convenience and healthcare providers with more comprehensive data for better management. Focusing on sustainable product designs and eco-friendly materials will appeal to environmentally conscious consumers and companies.
Major Players in the Europe Incontinence Devices and Ostomy Industry Ecosystem
- Unicharm Corporation
- Abena AS
- ConvaTec
- Hollister Inc
- Kimberly-Clark Corporation
- B Braun Melsungen AG
- Salts Healthcare
Key Developments in Europe Incontinence Devices and Ostomy Industry Industry
- June 2022: Ontex launched the Orizon digital incontinence management service, integrating sensor technology and a mobile application for improved patient monitoring and care management. This launch signals a significant shift towards digitally enabled incontinence care.
- March 2022: The European Investment Bank (EIB) invested USD 15.8 Million in Innovacell, highlighting increased investment in technological advancements within the incontinence treatment space. This investment focuses specifically on fecal and urinary incontinence conditions, indicating a future push towards innovative solutions.
Strategic Europe Incontinence Devices and Ostomy Industry Market Forecast
The European incontinence devices and ostomy market is projected to experience robust growth, driven by several key factors. These include the aging population across Europe, the rising prevalence of chronic diseases that often lead to incontinence and ostomy needs, and continuous technological advancements resulting in more effective and comfortable products. The increasing adoption of telehealth solutions and the development of more comfortable and discreet products will further stimulate market expansion. The market is expected to witness significant increases in demand for specialized and high-performance products, particularly those incorporating digital technologies for remote monitoring and personalized care. This positive outlook presents substantial opportunities for established companies and emerging players alike. The report provides detailed market forecasts, segmented by product type, application, and geography, offering a valuable resource for strategic planning and investment decisions.
Europe Incontinence Devices and Ostomy Industry Segmentation
-
1. Product Type
-
1.1. Incontinence Care Products
- 1.1.1. Absorbents
- 1.1.2. Incontinence Bags
- 1.1.3. Other Product Types
-
1.2. Ostomy Care Products
-
1.2.1. Ostomy Bags
- 1.2.1.1. Colostomy Bags
- 1.2.1.2. Ileostomy Bags
- 1.2.1.3. Urostomy Bags
- 1.2.2. Skin Barriers
- 1.2.3. Irrigation Products
- 1.2.4. Other Ostomy Products
-
1.2.1. Ostomy Bags
-
1.1. Incontinence Care Products
-
2. Application
- 2.1. Bladder Cancer
- 2.2. Colorectal Cancer
- 2.3. Crohn's Disease
- 2.4. Kidney Stone
- 2.5. Chronic Kidney Failure
- 2.6. Other Applications
Europe Incontinence Devices and Ostomy Industry Segmentation By Geography
- 1. Germany
- 2. United Kingdom
- 3. France
- 4. Spain
- 5. Italy
- 6. Rest of Europe
Europe Incontinence Devices and Ostomy Industry REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 10.10% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Rising Geriatric and Obese Populations; Increasing Prevalence of Renal Diseases and Nephrological Injuries
- 3.3. Market Restrains
- 3.3.1. Lack of Proper Reimbursement; Complications Associated with Ostomy and Usage of Incontinence Products
- 3.4. Market Trends
- 3.4.1. Colorectal Cancer is Expected to Hold a Significant Share in the Growth of the Market Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Europe Incontinence Devices and Ostomy Industry Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Product Type
- 5.1.1. Incontinence Care Products
- 5.1.1.1. Absorbents
- 5.1.1.2. Incontinence Bags
- 5.1.1.3. Other Product Types
- 5.1.2. Ostomy Care Products
- 5.1.2.1. Ostomy Bags
- 5.1.2.1.1. Colostomy Bags
- 5.1.2.1.2. Ileostomy Bags
- 5.1.2.1.3. Urostomy Bags
- 5.1.2.2. Skin Barriers
- 5.1.2.3. Irrigation Products
- 5.1.2.4. Other Ostomy Products
- 5.1.2.1. Ostomy Bags
- 5.1.1. Incontinence Care Products
- 5.2. Market Analysis, Insights and Forecast - by Application
- 5.2.1. Bladder Cancer
- 5.2.2. Colorectal Cancer
- 5.2.3. Crohn's Disease
- 5.2.4. Kidney Stone
- 5.2.5. Chronic Kidney Failure
- 5.2.6. Other Applications
- 5.3. Market Analysis, Insights and Forecast - by Region
- 5.3.1. Germany
- 5.3.2. United Kingdom
- 5.3.3. France
- 5.3.4. Spain
- 5.3.5. Italy
- 5.3.6. Rest of Europe
- 5.1. Market Analysis, Insights and Forecast - by Product Type
- 6. Germany Europe Incontinence Devices and Ostomy Industry Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Product Type
- 6.1.1. Incontinence Care Products
- 6.1.1.1. Absorbents
- 6.1.1.2. Incontinence Bags
- 6.1.1.3. Other Product Types
- 6.1.2. Ostomy Care Products
- 6.1.2.1. Ostomy Bags
- 6.1.2.1.1. Colostomy Bags
- 6.1.2.1.2. Ileostomy Bags
- 6.1.2.1.3. Urostomy Bags
- 6.1.2.2. Skin Barriers
- 6.1.2.3. Irrigation Products
- 6.1.2.4. Other Ostomy Products
- 6.1.2.1. Ostomy Bags
- 6.1.1. Incontinence Care Products
- 6.2. Market Analysis, Insights and Forecast - by Application
- 6.2.1. Bladder Cancer
- 6.2.2. Colorectal Cancer
- 6.2.3. Crohn's Disease
- 6.2.4. Kidney Stone
- 6.2.5. Chronic Kidney Failure
- 6.2.6. Other Applications
- 6.1. Market Analysis, Insights and Forecast - by Product Type
- 7. United Kingdom Europe Incontinence Devices and Ostomy Industry Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Product Type
- 7.1.1. Incontinence Care Products
- 7.1.1.1. Absorbents
- 7.1.1.2. Incontinence Bags
- 7.1.1.3. Other Product Types
- 7.1.2. Ostomy Care Products
- 7.1.2.1. Ostomy Bags
- 7.1.2.1.1. Colostomy Bags
- 7.1.2.1.2. Ileostomy Bags
- 7.1.2.1.3. Urostomy Bags
- 7.1.2.2. Skin Barriers
- 7.1.2.3. Irrigation Products
- 7.1.2.4. Other Ostomy Products
- 7.1.2.1. Ostomy Bags
- 7.1.1. Incontinence Care Products
- 7.2. Market Analysis, Insights and Forecast - by Application
- 7.2.1. Bladder Cancer
- 7.2.2. Colorectal Cancer
- 7.2.3. Crohn's Disease
- 7.2.4. Kidney Stone
- 7.2.5. Chronic Kidney Failure
- 7.2.6. Other Applications
- 7.1. Market Analysis, Insights and Forecast - by Product Type
- 8. France Europe Incontinence Devices and Ostomy Industry Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Product Type
- 8.1.1. Incontinence Care Products
- 8.1.1.1. Absorbents
- 8.1.1.2. Incontinence Bags
- 8.1.1.3. Other Product Types
- 8.1.2. Ostomy Care Products
- 8.1.2.1. Ostomy Bags
- 8.1.2.1.1. Colostomy Bags
- 8.1.2.1.2. Ileostomy Bags
- 8.1.2.1.3. Urostomy Bags
- 8.1.2.2. Skin Barriers
- 8.1.2.3. Irrigation Products
- 8.1.2.4. Other Ostomy Products
- 8.1.2.1. Ostomy Bags
- 8.1.1. Incontinence Care Products
- 8.2. Market Analysis, Insights and Forecast - by Application
- 8.2.1. Bladder Cancer
- 8.2.2. Colorectal Cancer
- 8.2.3. Crohn's Disease
- 8.2.4. Kidney Stone
- 8.2.5. Chronic Kidney Failure
- 8.2.6. Other Applications
- 8.1. Market Analysis, Insights and Forecast - by Product Type
- 9. Spain Europe Incontinence Devices and Ostomy Industry Analysis, Insights and Forecast, 2019-2031
- 9.1. Market Analysis, Insights and Forecast - by Product Type
- 9.1.1. Incontinence Care Products
- 9.1.1.1. Absorbents
- 9.1.1.2. Incontinence Bags
- 9.1.1.3. Other Product Types
- 9.1.2. Ostomy Care Products
- 9.1.2.1. Ostomy Bags
- 9.1.2.1.1. Colostomy Bags
- 9.1.2.1.2. Ileostomy Bags
- 9.1.2.1.3. Urostomy Bags
- 9.1.2.2. Skin Barriers
- 9.1.2.3. Irrigation Products
- 9.1.2.4. Other Ostomy Products
- 9.1.2.1. Ostomy Bags
- 9.1.1. Incontinence Care Products
- 9.2. Market Analysis, Insights and Forecast - by Application
- 9.2.1. Bladder Cancer
- 9.2.2. Colorectal Cancer
- 9.2.3. Crohn's Disease
- 9.2.4. Kidney Stone
- 9.2.5. Chronic Kidney Failure
- 9.2.6. Other Applications
- 9.1. Market Analysis, Insights and Forecast - by Product Type
- 10. Italy Europe Incontinence Devices and Ostomy Industry Analysis, Insights and Forecast, 2019-2031
- 10.1. Market Analysis, Insights and Forecast - by Product Type
- 10.1.1. Incontinence Care Products
- 10.1.1.1. Absorbents
- 10.1.1.2. Incontinence Bags
- 10.1.1.3. Other Product Types
- 10.1.2. Ostomy Care Products
- 10.1.2.1. Ostomy Bags
- 10.1.2.1.1. Colostomy Bags
- 10.1.2.1.2. Ileostomy Bags
- 10.1.2.1.3. Urostomy Bags
- 10.1.2.2. Skin Barriers
- 10.1.2.3. Irrigation Products
- 10.1.2.4. Other Ostomy Products
- 10.1.2.1. Ostomy Bags
- 10.1.1. Incontinence Care Products
- 10.2. Market Analysis, Insights and Forecast - by Application
- 10.2.1. Bladder Cancer
- 10.2.2. Colorectal Cancer
- 10.2.3. Crohn's Disease
- 10.2.4. Kidney Stone
- 10.2.5. Chronic Kidney Failure
- 10.2.6. Other Applications
- 10.1. Market Analysis, Insights and Forecast - by Product Type
- 11. Rest of Europe Europe Incontinence Devices and Ostomy Industry Analysis, Insights and Forecast, 2019-2031
- 11.1. Market Analysis, Insights and Forecast - by Product Type
- 11.1.1. Incontinence Care Products
- 11.1.1.1. Absorbents
- 11.1.1.2. Incontinence Bags
- 11.1.1.3. Other Product Types
- 11.1.2. Ostomy Care Products
- 11.1.2.1. Ostomy Bags
- 11.1.2.1.1. Colostomy Bags
- 11.1.2.1.2. Ileostomy Bags
- 11.1.2.1.3. Urostomy Bags
- 11.1.2.2. Skin Barriers
- 11.1.2.3. Irrigation Products
- 11.1.2.4. Other Ostomy Products
- 11.1.2.1. Ostomy Bags
- 11.1.1. Incontinence Care Products
- 11.2. Market Analysis, Insights and Forecast - by Application
- 11.2.1. Bladder Cancer
- 11.2.2. Colorectal Cancer
- 11.2.3. Crohn's Disease
- 11.2.4. Kidney Stone
- 11.2.5. Chronic Kidney Failure
- 11.2.6. Other Applications
- 11.1. Market Analysis, Insights and Forecast - by Product Type
- 12. Germany Europe Incontinence Devices and Ostomy Industry Analysis, Insights and Forecast, 2019-2031
- 13. France Europe Incontinence Devices and Ostomy Industry Analysis, Insights and Forecast, 2019-2031
- 14. Italy Europe Incontinence Devices and Ostomy Industry Analysis, Insights and Forecast, 2019-2031
- 15. United Kingdom Europe Incontinence Devices and Ostomy Industry Analysis, Insights and Forecast, 2019-2031
- 16. Netherlands Europe Incontinence Devices and Ostomy Industry Analysis, Insights and Forecast, 2019-2031
- 17. Sweden Europe Incontinence Devices and Ostomy Industry Analysis, Insights and Forecast, 2019-2031
- 18. Rest of Europe Europe Incontinence Devices and Ostomy Industry Analysis, Insights and Forecast, 2019-2031
- 19. Competitive Analysis
- 19.1. Market Share Analysis 2024
- 19.2. Company Profiles
- 19.2.1 Unicharm Corporation
- 19.2.1.1. Overview
- 19.2.1.2. Products
- 19.2.1.3. SWOT Analysis
- 19.2.1.4. Recent Developments
- 19.2.1.5. Financials (Based on Availability)
- 19.2.2 Abena AS
- 19.2.2.1. Overview
- 19.2.2.2. Products
- 19.2.2.3. SWOT Analysis
- 19.2.2.4. Recent Developments
- 19.2.2.5. Financials (Based on Availability)
- 19.2.3 ConvaTec
- 19.2.3.1. Overview
- 19.2.3.2. Products
- 19.2.3.3. SWOT Analysis
- 19.2.3.4. Recent Developments
- 19.2.3.5. Financials (Based on Availability)
- 19.2.4 Hollister Inc
- 19.2.4.1. Overview
- 19.2.4.2. Products
- 19.2.4.3. SWOT Analysis
- 19.2.4.4. Recent Developments
- 19.2.4.5. Financials (Based on Availability)
- 19.2.5 Kimberly-Clark Corporation
- 19.2.5.1. Overview
- 19.2.5.2. Products
- 19.2.5.3. SWOT Analysis
- 19.2.5.4. Recent Developments
- 19.2.5.5. Financials (Based on Availability)
- 19.2.6 B Braun Melsungen AG
- 19.2.6.1. Overview
- 19.2.6.2. Products
- 19.2.6.3. SWOT Analysis
- 19.2.6.4. Recent Developments
- 19.2.6.5. Financials (Based on Availability)
- 19.2.7 Salts Healthcare
- 19.2.7.1. Overview
- 19.2.7.2. Products
- 19.2.7.3. SWOT Analysis
- 19.2.7.4. Recent Developments
- 19.2.7.5. Financials (Based on Availability)
- 19.2.1 Unicharm Corporation
List of Figures
- Figure 1: Europe Incontinence Devices and Ostomy Industry Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Europe Incontinence Devices and Ostomy Industry Share (%) by Company 2024
List of Tables
- Table 1: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 3: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 4: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Region 2019 & 2032
- Table 5: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 6: Germany Europe Incontinence Devices and Ostomy Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 7: France Europe Incontinence Devices and Ostomy Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 8: Italy Europe Incontinence Devices and Ostomy Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 9: United Kingdom Europe Incontinence Devices and Ostomy Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 10: Netherlands Europe Incontinence Devices and Ostomy Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 11: Sweden Europe Incontinence Devices and Ostomy Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 12: Rest of Europe Europe Incontinence Devices and Ostomy Industry Revenue (Million) Forecast, by Application 2019 & 2032
- Table 13: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 14: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 15: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 16: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 17: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 18: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 19: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 20: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 21: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 22: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 23: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 24: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 25: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 26: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 27: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Country 2019 & 2032
- Table 28: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Product Type 2019 & 2032
- Table 29: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Application 2019 & 2032
- Table 30: Europe Incontinence Devices and Ostomy Industry Revenue Million Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Europe Incontinence Devices and Ostomy Industry?
The projected CAGR is approximately 10.10%.
2. Which companies are prominent players in the Europe Incontinence Devices and Ostomy Industry?
Key companies in the market include Unicharm Corporation, Abena AS, ConvaTec, Hollister Inc, Kimberly-Clark Corporation, B Braun Melsungen AG, Salts Healthcare.
3. What are the main segments of the Europe Incontinence Devices and Ostomy Industry?
The market segments include Product Type, Application.
4. Can you provide details about the market size?
The market size is estimated to be USD XX Million as of 2022.
5. What are some drivers contributing to market growth?
Rising Geriatric and Obese Populations; Increasing Prevalence of Renal Diseases and Nephrological Injuries.
6. What are the notable trends driving market growth?
Colorectal Cancer is Expected to Hold a Significant Share in the Growth of the Market Over the Forecast Period.
7. Are there any restraints impacting market growth?
Lack of Proper Reimbursement; Complications Associated with Ostomy and Usage of Incontinence Products.
8. Can you provide examples of recent developments in the market?
June 2022: Ontex launched the Orizon digital incontinence management service in late 2022 or early 2023. The solution contains printed sensors, transmitters clipped onto diapers, and a mobile and web application.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Europe Incontinence Devices and Ostomy Industry," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Europe Incontinence Devices and Ostomy Industry report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Europe Incontinence Devices and Ostomy Industry?
To stay informed about further developments, trends, and reports in the Europe Incontinence Devices and Ostomy Industry, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


