Key Insights
The North American blood screening market, valued at $7.15 billion in 2025, is projected to experience robust growth, driven by several key factors. The increasing prevalence of chronic diseases like colorectal cancer and cardiovascular disease necessitates frequent and comprehensive screening, fueling demand for advanced blood tests. Technological advancements, such as the development of more sensitive and specific assays, coupled with the adoption of point-of-care diagnostics, are further boosting market expansion. Government initiatives promoting preventive healthcare and early disease detection also contribute significantly. The market is segmented by screening test type (stool-based tests, stool DNA tests, colonoscopy, CT colonography, flexible sigmoidoscopy, and other products) and end-user (hospitals, diagnostic centers/laboratories, and other end-users). Competition is fierce, with major players like Abbott Laboratories, Roche, and Exact Sciences Corporation vying for market share through innovation and strategic partnerships. While the high cost of advanced screening technologies and potential reimbursement challenges pose some restraints, the overall market outlook remains positive, driven by an aging population and rising awareness about preventive healthcare.
The significant growth trajectory of the North American blood screening market is expected to continue through 2033, propelled by the projected increase in the geriatric population, which is particularly susceptible to various diseases necessitating regular screening. Furthermore, the market is poised to benefit from the continuous refinement of existing technologies and the introduction of novel screening methods offering higher accuracy, faster results, and improved patient experience. The expanding healthcare infrastructure and the rising investment in research and development within the diagnostics sector further contribute to the market's expansion. However, potential challenges include ensuring equitable access to advanced screening across diverse socioeconomic groups and navigating the regulatory landscape surrounding new diagnostic technologies. Despite these challenges, the long-term growth prospects for the North American blood screening market are exceptionally promising, making it an attractive sector for both established players and emerging innovators.
Blood Screening Industry in North America: A Comprehensive Market Analysis (2019-2033)
This insightful report provides a comprehensive analysis of the North American blood screening industry, offering a detailed examination of market trends, key players, and future growth prospects. The study covers the period from 2019 to 2033, with 2025 serving as the base and estimated year. It delves into various segments, including stool-based tests, stool DNA tests, and different end-users, providing valuable insights for stakeholders seeking to understand and capitalize on this dynamic market. The total market size in 2025 is estimated at $xx Million.
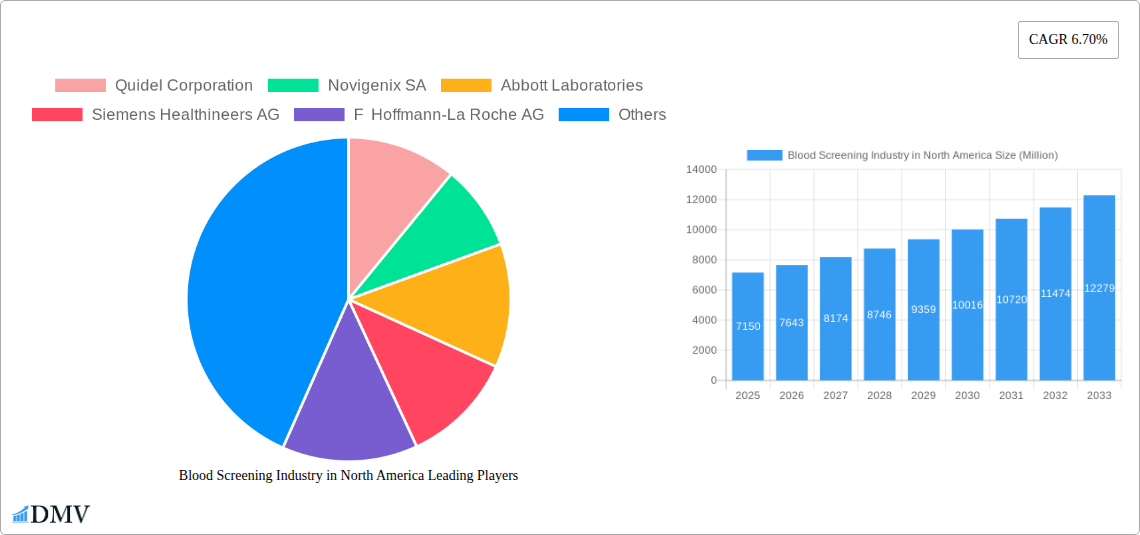
Blood Screening Industry in North America Market Composition & Trends
This section analyzes the competitive landscape of the North American blood screening market, evaluating market concentration, innovation drivers, regulatory frameworks, substitute products, and end-user profiles. We examine the landscape of mergers and acquisitions (M&A) activity within the sector, including deal values and their impact on market share distribution.
Market Concentration: The North American blood screening market exhibits a moderately concentrated structure, with a handful of large multinational corporations holding significant market share. However, smaller companies specializing in niche areas are also present, fostering innovation. The combined market share of the top five players in 2025 is estimated at 60%.
Innovation Catalysts: Technological advancements, such as advancements in molecular diagnostics and liquid biopsies, are driving innovation, leading to the development of more sensitive and specific blood-based screening tests. The increasing adoption of artificial intelligence (AI) and machine learning in test interpretation is another key catalyst.
Regulatory Landscape: The regulatory environment plays a crucial role, with agencies like the FDA in the United States influencing product approvals and market access. Navigating these regulatory hurdles is critical for market entry and sustained growth.
Substitute Products: While blood-based screening tests offer several advantages, they face competition from traditional screening methods such as colonoscopy and other imaging techniques. The choice often depends on factors like patient preference, cost, and the invasiveness of the procedure.
End-User Profiles: The primary end-users include hospitals, diagnostic centers/laboratories, and other healthcare providers. The market segmentation shows hospitals accounting for the largest share due to their extensive testing capacity. The preference for blood-based tests over traditional methods also contributes to growth in certain areas.
M&A Activity: The blood screening industry has witnessed significant M&A activity in recent years, with deal values exceeding $xx Million in the last five years. These activities aim to consolidate market share and enhance technological capabilities. Key examples include [insert specific examples of relevant M&A deals and their values].
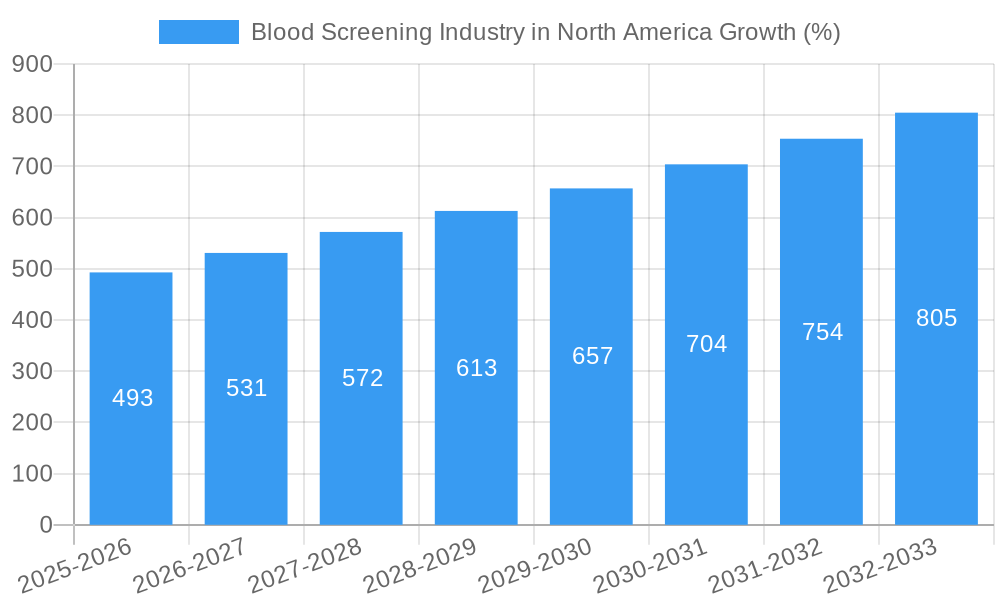
Blood Screening Industry in North America Industry Evolution
The North American blood screening market has experienced substantial growth over the past five years (2019-2024), driven by factors such as increasing prevalence of chronic diseases, technological advancements resulting in more accurate and convenient tests, and rising healthcare expenditure. We project a compound annual growth rate (CAGR) of xx% from 2025 to 2033.
This growth trajectory is further propelled by several significant developments: the increasing adoption of preventative healthcare measures, advancements in liquid biopsy technologies offering earlier and more accurate detection of diseases, and the growing preference for non-invasive screening methods amongst consumers. The rise of personalized medicine, targeting specific genetic predispositions to certain diseases, is another significant driver.
Technological advancements have significantly improved the sensitivity and specificity of blood screening tests, leading to earlier disease detection and better patient outcomes. This has improved compliance among at-risk populations and reduced the reliance on more invasive and time-consuming procedures. The development of point-of-care testing (POCT) devices also allows for faster turnaround times and increased accessibility.
Consumer demand is evolving, with a greater emphasis on convenience, affordability, and non-invasive screening options. These preferences are driving the industry to develop new tests with improved user-friendliness and shorter turnaround times. The rising awareness of chronic diseases and the desire for proactive health management are major contributing factors to this shift in consumer demand. The increasing accessibility of genetic testing and personalized medicine options is also influencing consumer preferences and shaping the industry's future direction.
Leading Regions, Countries, or Segments in Blood Screening Industry in North America
The United States dominates the North American blood screening market, driven by several factors: high healthcare expenditure, advanced healthcare infrastructure, and a larger at-risk population. Canada also holds a significant market share, although smaller than the US.
Key Drivers for US Dominance:
- High healthcare spending per capita
- Strong regulatory support for innovative screening technologies
- Advanced healthcare infrastructure and well-established diagnostic networks.
- Early adoption of new technologies.
Segment Analysis:
- Stool-based tests: While traditional stool-based tests remain prevalent, their market share is gradually declining due to the emergence of more advanced and convenient blood-based tests.
- Stool DNA tests: This segment is experiencing substantial growth, with improved accuracy and ease of use making them attractive alternatives to colonoscopies.
- Colonoscopy: Colonoscopy remains a standard procedure despite the growth in other screening methods due to its high sensitivity and ability to perform biopsies.
- CT Colonography: This imaging technique offers a less-invasive alternative to colonoscopy, and its market is seeing moderate growth.
- Flexible Sigmoidoscopy: This procedure's market share is relatively stable, often used in conjunction with other screening methods.
- Other Products: This segment encompasses emerging and innovative screening technologies, representing a significant growth opportunity.
- Hospitals: Hospitals remain the largest end-users due to their extensive infrastructure and capacity for sophisticated testing.
- Diagnostic Centers/Laboratories: Independent laboratories are key players, providing outsourced testing services.
- Other End-Users: This includes physician offices and other healthcare facilities, contributing to the overall market growth.
Blood Screening Industry in North America Product Innovations
Significant advancements in blood screening technologies are revolutionizing early disease detection. Liquid biopsies, capable of detecting circulating tumor DNA (ctDNA) and other biomarkers, are gaining traction, allowing for earlier and more accurate diagnosis of various cancers. Miniaturized devices and point-of-care testing platforms enable faster turnaround times and improved accessibility, particularly in remote areas. These innovations offer enhanced sensitivity, specificity, and convenience compared to traditional screening methods, resulting in improved patient outcomes and cost-effectiveness. The development of multiplex assays, which simultaneously detect multiple biomarkers, further enhances the diagnostic capabilities of blood-based screening tests.
Propelling Factors for Blood Screening Industry in North America Growth
Several factors contribute to the growth of the North American blood screening industry. The rising prevalence of chronic diseases like cancer and cardiovascular diseases necessitates more effective screening methods. Technological advancements, such as liquid biopsies and improved diagnostic tools, provide more accurate and less invasive options. Government initiatives promoting preventative healthcare and increased healthcare spending further stimulate market growth. The increasing awareness among the population about early disease detection and proactive health management is also fueling the demand for blood screening services.
Obstacles in the Blood Screening Industry in North America Market
Despite the significant growth potential, the industry faces certain challenges. High costs associated with developing and implementing new technologies can limit access. Strict regulatory processes for gaining FDA approval can delay product launches and market entry. Competition from established screening methods, like colonoscopies, continues to exert pressure. Furthermore, potential supply chain disruptions, especially concerning reagents and consumables, may impact market stability. These challenges need to be addressed effectively to ensure sustainable growth.
Future Opportunities in Blood Screening Industry in North America
The future of the North American blood screening industry appears promising. The development of new biomarkers and more sophisticated diagnostic tools will enhance the accuracy and capabilities of existing tests. Expansion into new therapeutic areas beyond cancer detection, such as cardiovascular disease screening, presents significant growth opportunities. Personalized medicine approaches, tailoring screening strategies based on individual genetic profiles, will gain traction. The integration of AI and machine learning into test interpretation and risk assessment will further improve diagnostic accuracy and efficiency, shaping the future of preventative healthcare.
Major Players in the Blood Screening Industry in North America Ecosystem
- Quidel Corporation
- Novigenix SA
- Abbott Laboratories
- Siemens Healthineers AG
- F Hoffmann-La Roche AG
- Exact Sciences Corporation
- Clinical Genomics Technologies Pty Ltd
- EKF Diagnostics
- MAINZ BIOMED N V
- Sysmex Corporation
- Epigenomics Inc
- Hemosure Inc
Key Developments in Blood Screening Industry in North America Industry
December 2022: Guardant Health, Inc. reported positive results from the ECLIPSE study, a large-scale evaluation of its blood test for detecting colorectal cancer (CRC) in average-risk adults. This development signifies a significant advancement in early cancer detection.
February 2022: The FDA approved QIAGEN Manchester Ltd.'s Therascreen KRAS RGQ PCR Kit for colorectal cancer screening, broadening the availability of molecular diagnostic tools for CRC detection.
Strategic Blood Screening Industry in North America Market Forecast
The North American blood screening market is poised for robust growth, driven by continuous technological advancements, increased awareness of preventative healthcare, and the rising prevalence of chronic diseases. The adoption of innovative technologies like liquid biopsies and point-of-care testing will significantly shape market dynamics. The expanding pipeline of new blood-based screening tests for various diseases will further fuel market expansion. The integration of AI and big data will enhance diagnostic accuracy, creating a more efficient and personalized screening approach, ultimately leading to improved patient outcomes and market growth across the forecast period (2025-2033).
Blood Screening Industry in North America Segmentation
-
1. Screening Tests
-
1.1. Stool-based Tests
- 1.1.1. Fecal Immunochemical Test (FIT)
- 1.1.2. Guaiac-based Fecal Occult Blood Test (gFOBT)
- 1.1.3. Stool DNA Test
- 1.2. Colonoscopy
- 1.3. CT Colonography (Virtual Colonoscopy)
- 1.4. Flexible Sigmoidoscopy
- 1.5. Other Screening Tests
-
1.1. Stool-based Tests
-
2. End User
- 2.1. Hospitals
- 2.2. Diagnostic Centers/Laboratories
- 2.3. Other End Users
-
3. Geography
- 3.1. United States
- 3.2. Canada
- 3.3. Mexico
Blood Screening Industry in North America Segmentation By Geography
- 1. United States
- 2. Canada
- 3. Mexico
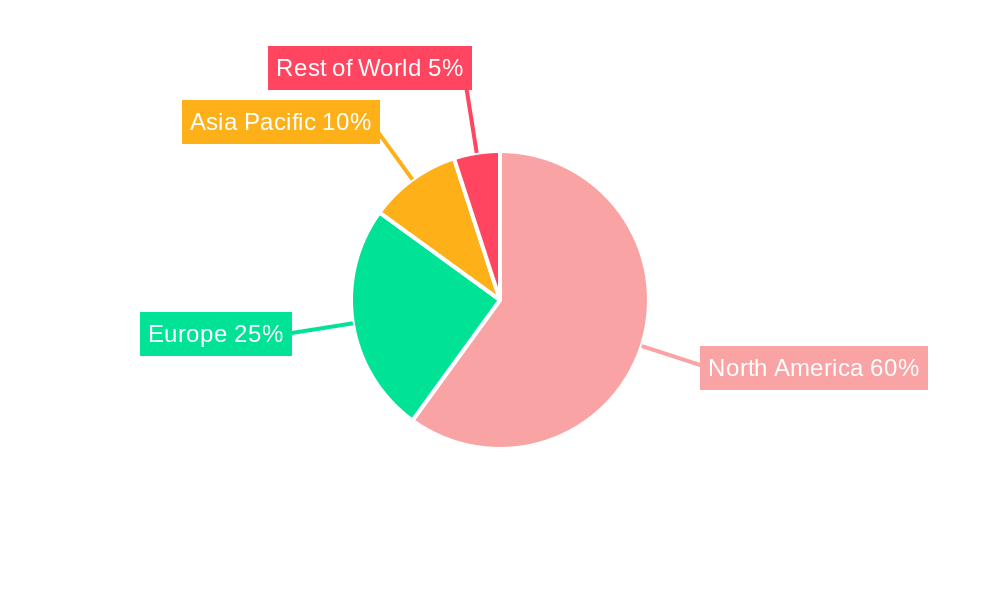
Blood Screening Industry in North America REPORT HIGHLIGHTS
| Aspects | Details |
|---|---|
| Study Period | 2019-2033 |
| Base Year | 2024 |
| Estimated Year | 2025 |
| Forecast Period | 2025-2033 |
| Historical Period | 2019-2024 |
| Growth Rate | CAGR of 6.70% from 2019-2033 |
| Segmentation |
|
Table of Contents
- 1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
- 2. Executive Summary
- 2.1. Introduction
- 3. Market Dynamics
- 3.1. Introduction
- 3.2. Market Drivers
- 3.2.1. Increasing Prevalence of Colorectal Cancer; Technological advancements and Increasing Cancer Prevention Initiatives
- 3.3. Market Restrains
- 3.3.1. High Screening Tests Costs
- 3.4. Market Trends
- 3.4.1. Guaiac Fecal Occult Blood Test (gFOBT) is Expected to be Hold a Significant Market Share Over the Forecast Period
- 4. Market Factor Analysis
- 4.1. Porters Five Forces
- 4.2. Supply/Value Chain
- 4.3. PESTEL analysis
- 4.4. Market Entropy
- 4.5. Patent/Trademark Analysis
- 5. Blood Screening Industry in North America Analysis, Insights and Forecast, 2019-2031
- 5.1. Market Analysis, Insights and Forecast - by Screening Tests
- 5.1.1. Stool-based Tests
- 5.1.1.1. Fecal Immunochemical Test (FIT)
- 5.1.1.2. Guaiac-based Fecal Occult Blood Test (gFOBT)
- 5.1.1.3. Stool DNA Test
- 5.1.2. Colonoscopy
- 5.1.3. CT Colonography (Virtual Colonoscopy)
- 5.1.4. Flexible Sigmoidoscopy
- 5.1.5. Other Screening Tests
- 5.1.1. Stool-based Tests
- 5.2. Market Analysis, Insights and Forecast - by End User
- 5.2.1. Hospitals
- 5.2.2. Diagnostic Centers/Laboratories
- 5.2.3. Other End Users
- 5.3. Market Analysis, Insights and Forecast - by Geography
- 5.3.1. United States
- 5.3.2. Canada
- 5.3.3. Mexico
- 5.4. Market Analysis, Insights and Forecast - by Region
- 5.4.1. United States
- 5.4.2. Canada
- 5.4.3. Mexico
- 5.1. Market Analysis, Insights and Forecast - by Screening Tests
- 6. United States Blood Screening Industry in North America Analysis, Insights and Forecast, 2019-2031
- 6.1. Market Analysis, Insights and Forecast - by Screening Tests
- 6.1.1. Stool-based Tests
- 6.1.1.1. Fecal Immunochemical Test (FIT)
- 6.1.1.2. Guaiac-based Fecal Occult Blood Test (gFOBT)
- 6.1.1.3. Stool DNA Test
- 6.1.2. Colonoscopy
- 6.1.3. CT Colonography (Virtual Colonoscopy)
- 6.1.4. Flexible Sigmoidoscopy
- 6.1.5. Other Screening Tests
- 6.1.1. Stool-based Tests
- 6.2. Market Analysis, Insights and Forecast - by End User
- 6.2.1. Hospitals
- 6.2.2. Diagnostic Centers/Laboratories
- 6.2.3. Other End Users
- 6.3. Market Analysis, Insights and Forecast - by Geography
- 6.3.1. United States
- 6.3.2. Canada
- 6.3.3. Mexico
- 6.1. Market Analysis, Insights and Forecast - by Screening Tests
- 7. Canada Blood Screening Industry in North America Analysis, Insights and Forecast, 2019-2031
- 7.1. Market Analysis, Insights and Forecast - by Screening Tests
- 7.1.1. Stool-based Tests
- 7.1.1.1. Fecal Immunochemical Test (FIT)
- 7.1.1.2. Guaiac-based Fecal Occult Blood Test (gFOBT)
- 7.1.1.3. Stool DNA Test
- 7.1.2. Colonoscopy
- 7.1.3. CT Colonography (Virtual Colonoscopy)
- 7.1.4. Flexible Sigmoidoscopy
- 7.1.5. Other Screening Tests
- 7.1.1. Stool-based Tests
- 7.2. Market Analysis, Insights and Forecast - by End User
- 7.2.1. Hospitals
- 7.2.2. Diagnostic Centers/Laboratories
- 7.2.3. Other End Users
- 7.3. Market Analysis, Insights and Forecast - by Geography
- 7.3.1. United States
- 7.3.2. Canada
- 7.3.3. Mexico
- 7.1. Market Analysis, Insights and Forecast - by Screening Tests
- 8. Mexico Blood Screening Industry in North America Analysis, Insights and Forecast, 2019-2031
- 8.1. Market Analysis, Insights and Forecast - by Screening Tests
- 8.1.1. Stool-based Tests
- 8.1.1.1. Fecal Immunochemical Test (FIT)
- 8.1.1.2. Guaiac-based Fecal Occult Blood Test (gFOBT)
- 8.1.1.3. Stool DNA Test
- 8.1.2. Colonoscopy
- 8.1.3. CT Colonography (Virtual Colonoscopy)
- 8.1.4. Flexible Sigmoidoscopy
- 8.1.5. Other Screening Tests
- 8.1.1. Stool-based Tests
- 8.2. Market Analysis, Insights and Forecast - by End User
- 8.2.1. Hospitals
- 8.2.2. Diagnostic Centers/Laboratories
- 8.2.3. Other End Users
- 8.3. Market Analysis, Insights and Forecast - by Geography
- 8.3.1. United States
- 8.3.2. Canada
- 8.3.3. Mexico
- 8.1. Market Analysis, Insights and Forecast - by Screening Tests
- 9. United States Blood Screening Industry in North America Analysis, Insights and Forecast, 2019-2031
- 10. Canada Blood Screening Industry in North America Analysis, Insights and Forecast, 2019-2031
- 11. Mexico Blood Screening Industry in North America Analysis, Insights and Forecast, 2019-2031
- 12. Rest of North America Blood Screening Industry in North America Analysis, Insights and Forecast, 2019-2031
- 13. Competitive Analysis
- 13.1. Market Share Analysis 2024
- 13.2. Company Profiles
- 13.2.1 Quidel Corporation
- 13.2.1.1. Overview
- 13.2.1.2. Products
- 13.2.1.3. SWOT Analysis
- 13.2.1.4. Recent Developments
- 13.2.1.5. Financials (Based on Availability)
- 13.2.2 Novigenix SA
- 13.2.2.1. Overview
- 13.2.2.2. Products
- 13.2.2.3. SWOT Analysis
- 13.2.2.4. Recent Developments
- 13.2.2.5. Financials (Based on Availability)
- 13.2.3 Abbott Laboratories
- 13.2.3.1. Overview
- 13.2.3.2. Products
- 13.2.3.3. SWOT Analysis
- 13.2.3.4. Recent Developments
- 13.2.3.5. Financials (Based on Availability)
- 13.2.4 Siemens Healthineers AG
- 13.2.4.1. Overview
- 13.2.4.2. Products
- 13.2.4.3. SWOT Analysis
- 13.2.4.4. Recent Developments
- 13.2.4.5. Financials (Based on Availability)
- 13.2.5 F Hoffmann-La Roche AG
- 13.2.5.1. Overview
- 13.2.5.2. Products
- 13.2.5.3. SWOT Analysis
- 13.2.5.4. Recent Developments
- 13.2.5.5. Financials (Based on Availability)
- 13.2.6 Exact Sciences Corporation
- 13.2.6.1. Overview
- 13.2.6.2. Products
- 13.2.6.3. SWOT Analysis
- 13.2.6.4. Recent Developments
- 13.2.6.5. Financials (Based on Availability)
- 13.2.7 Clinical Genomics Technologies Pty Ltd
- 13.2.7.1. Overview
- 13.2.7.2. Products
- 13.2.7.3. SWOT Analysis
- 13.2.7.4. Recent Developments
- 13.2.7.5. Financials (Based on Availability)
- 13.2.8 EKF Diagnostics
- 13.2.8.1. Overview
- 13.2.8.2. Products
- 13.2.8.3. SWOT Analysis
- 13.2.8.4. Recent Developments
- 13.2.8.5. Financials (Based on Availability)
- 13.2.9 MAINZ BIOMED N V
- 13.2.9.1. Overview
- 13.2.9.2. Products
- 13.2.9.3. SWOT Analysis
- 13.2.9.4. Recent Developments
- 13.2.9.5. Financials (Based on Availability)
- 13.2.10 Sysmex Corporation
- 13.2.10.1. Overview
- 13.2.10.2. Products
- 13.2.10.3. SWOT Analysis
- 13.2.10.4. Recent Developments
- 13.2.10.5. Financials (Based on Availability)
- 13.2.11 Epigenomics Inc
- 13.2.11.1. Overview
- 13.2.11.2. Products
- 13.2.11.3. SWOT Analysis
- 13.2.11.4. Recent Developments
- 13.2.11.5. Financials (Based on Availability)
- 13.2.12 Hemosure Inc
- 13.2.12.1. Overview
- 13.2.12.2. Products
- 13.2.12.3. SWOT Analysis
- 13.2.12.4. Recent Developments
- 13.2.12.5. Financials (Based on Availability)
- 13.2.1 Quidel Corporation
List of Figures
- Figure 1: Blood Screening Industry in North America Revenue Breakdown (Million, %) by Product 2024 & 2032
- Figure 2: Blood Screening Industry in North America Share (%) by Company 2024
List of Tables
- Table 1: Blood Screening Industry in North America Revenue Million Forecast, by Region 2019 & 2032
- Table 2: Blood Screening Industry in North America Volume K Unit Forecast, by Region 2019 & 2032
- Table 3: Blood Screening Industry in North America Revenue Million Forecast, by Screening Tests 2019 & 2032
- Table 4: Blood Screening Industry in North America Volume K Unit Forecast, by Screening Tests 2019 & 2032
- Table 5: Blood Screening Industry in North America Revenue Million Forecast, by End User 2019 & 2032
- Table 6: Blood Screening Industry in North America Volume K Unit Forecast, by End User 2019 & 2032
- Table 7: Blood Screening Industry in North America Revenue Million Forecast, by Geography 2019 & 2032
- Table 8: Blood Screening Industry in North America Volume K Unit Forecast, by Geography 2019 & 2032
- Table 9: Blood Screening Industry in North America Revenue Million Forecast, by Region 2019 & 2032
- Table 10: Blood Screening Industry in North America Volume K Unit Forecast, by Region 2019 & 2032
- Table 11: Blood Screening Industry in North America Revenue Million Forecast, by Country 2019 & 2032
- Table 12: Blood Screening Industry in North America Volume K Unit Forecast, by Country 2019 & 2032
- Table 13: United States Blood Screening Industry in North America Revenue (Million) Forecast, by Application 2019 & 2032
- Table 14: United States Blood Screening Industry in North America Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 15: Canada Blood Screening Industry in North America Revenue (Million) Forecast, by Application 2019 & 2032
- Table 16: Canada Blood Screening Industry in North America Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 17: Mexico Blood Screening Industry in North America Revenue (Million) Forecast, by Application 2019 & 2032
- Table 18: Mexico Blood Screening Industry in North America Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 19: Rest of North America Blood Screening Industry in North America Revenue (Million) Forecast, by Application 2019 & 2032
- Table 20: Rest of North America Blood Screening Industry in North America Volume (K Unit) Forecast, by Application 2019 & 2032
- Table 21: Blood Screening Industry in North America Revenue Million Forecast, by Screening Tests 2019 & 2032
- Table 22: Blood Screening Industry in North America Volume K Unit Forecast, by Screening Tests 2019 & 2032
- Table 23: Blood Screening Industry in North America Revenue Million Forecast, by End User 2019 & 2032
- Table 24: Blood Screening Industry in North America Volume K Unit Forecast, by End User 2019 & 2032
- Table 25: Blood Screening Industry in North America Revenue Million Forecast, by Geography 2019 & 2032
- Table 26: Blood Screening Industry in North America Volume K Unit Forecast, by Geography 2019 & 2032
- Table 27: Blood Screening Industry in North America Revenue Million Forecast, by Country 2019 & 2032
- Table 28: Blood Screening Industry in North America Volume K Unit Forecast, by Country 2019 & 2032
- Table 29: Blood Screening Industry in North America Revenue Million Forecast, by Screening Tests 2019 & 2032
- Table 30: Blood Screening Industry in North America Volume K Unit Forecast, by Screening Tests 2019 & 2032
- Table 31: Blood Screening Industry in North America Revenue Million Forecast, by End User 2019 & 2032
- Table 32: Blood Screening Industry in North America Volume K Unit Forecast, by End User 2019 & 2032
- Table 33: Blood Screening Industry in North America Revenue Million Forecast, by Geography 2019 & 2032
- Table 34: Blood Screening Industry in North America Volume K Unit Forecast, by Geography 2019 & 2032
- Table 35: Blood Screening Industry in North America Revenue Million Forecast, by Country 2019 & 2032
- Table 36: Blood Screening Industry in North America Volume K Unit Forecast, by Country 2019 & 2032
- Table 37: Blood Screening Industry in North America Revenue Million Forecast, by Screening Tests 2019 & 2032
- Table 38: Blood Screening Industry in North America Volume K Unit Forecast, by Screening Tests 2019 & 2032
- Table 39: Blood Screening Industry in North America Revenue Million Forecast, by End User 2019 & 2032
- Table 40: Blood Screening Industry in North America Volume K Unit Forecast, by End User 2019 & 2032
- Table 41: Blood Screening Industry in North America Revenue Million Forecast, by Geography 2019 & 2032
- Table 42: Blood Screening Industry in North America Volume K Unit Forecast, by Geography 2019 & 2032
- Table 43: Blood Screening Industry in North America Revenue Million Forecast, by Country 2019 & 2032
- Table 44: Blood Screening Industry in North America Volume K Unit Forecast, by Country 2019 & 2032
Frequently Asked Questions
1. What is the projected Compound Annual Growth Rate (CAGR) of the Blood Screening Industry in North America?
The projected CAGR is approximately 6.70%.
2. Which companies are prominent players in the Blood Screening Industry in North America?
Key companies in the market include Quidel Corporation, Novigenix SA, Abbott Laboratories, Siemens Healthineers AG, F Hoffmann-La Roche AG, Exact Sciences Corporation, Clinical Genomics Technologies Pty Ltd, EKF Diagnostics, MAINZ BIOMED N V , Sysmex Corporation, Epigenomics Inc, Hemosure Inc.
3. What are the main segments of the Blood Screening Industry in North America?
The market segments include Screening Tests, End User, Geography.
4. Can you provide details about the market size?
The market size is estimated to be USD 7.15 Million as of 2022.
5. What are some drivers contributing to market growth?
Increasing Prevalence of Colorectal Cancer; Technological advancements and Increasing Cancer Prevention Initiatives.
6. What are the notable trends driving market growth?
Guaiac Fecal Occult Blood Test (gFOBT) is Expected to be Hold a Significant Market Share Over the Forecast Period.
7. Are there any restraints impacting market growth?
High Screening Tests Costs.
8. Can you provide examples of recent developments in the market?
December 2022: Guardant Health, Inc., a United States based Company, produced positive results from ECLIPSE (Evaluation of ctDNA LUNAR Assay In an Average Patient Screening Episode), an over 20,000 patient study for evaluating the performance of its blood test for detecting colorectal cancer (CRC) in average-risk adults.
9. What pricing options are available for accessing the report?
Pricing options include single-user, multi-user, and enterprise licenses priced at USD 4750, USD 5250, and USD 8750 respectively.
10. Is the market size provided in terms of value or volume?
The market size is provided in terms of value, measured in Million and volume, measured in K Unit.
11. Are there any specific market keywords associated with the report?
Yes, the market keyword associated with the report is "Blood Screening Industry in North America," which aids in identifying and referencing the specific market segment covered.
12. How do I determine which pricing option suits my needs best?
The pricing options vary based on user requirements and access needs. Individual users may opt for single-user licenses, while businesses requiring broader access may choose multi-user or enterprise licenses for cost-effective access to the report.
13. Are there any additional resources or data provided in the Blood Screening Industry in North America report?
While the report offers comprehensive insights, it's advisable to review the specific contents or supplementary materials provided to ascertain if additional resources or data are available.
14. How can I stay updated on further developments or reports in the Blood Screening Industry in North America?
To stay informed about further developments, trends, and reports in the Blood Screening Industry in North America, consider subscribing to industry newsletters, following relevant companies and organizations, or regularly checking reputable industry news sources and publications.
Methodology
Step 1 - Identification of Relevant Samples Size from Population Database



Step 2 - Approaches for Defining Global Market Size (Value, Volume* & Price*)

Note*: In applicable scenarios
Step 3 - Data Sources
Primary Research
- Web Analytics
- Survey Reports
- Research Institute
- Latest Research Reports
- Opinion Leaders
Secondary Research
- Annual Reports
- White Paper
- Latest Press Release
- Industry Association
- Paid Database
- Investor Presentations

Step 4 - Data Triangulation
Involves using different sources of information in order to increase the validity of a study
These sources are likely to be stakeholders in a program - participants, other researchers, program staff, other community members, and so on.
Then we put all data in single framework & apply various statistical tools to find out the dynamic on the market.
During the analysis stage, feedback from the stakeholder groups would be compared to determine areas of agreement as well as areas of divergence


